10-06-03 05:59
No 462963
Palladium-Catalyzed Reaction of Aryl Iodides with Acetic Anhydride. A Carbon Monoxide-Free Synthesis of Acetophenones
Sandro Cacchi,* Giancarlo Fabrizi, Federica Gavazza, and Antonella Goggiamani
Org. Lett., 5 (3), 289 -291, 2003 (http://pharmacist.the-hive.tripod.com/a
DOI:10.1021/ol027243b S1523-7060(02)07243-7
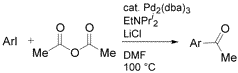
Abstract: The palladium-catalyzed reaction of aryl iodides with acetic anhydride provides a straightforward and experimentally simple carbon monoxide-free route to acetophenones. The reaction tolerates a wide range of functionalized aryl iodides. Acetophenones are isolated in excellent yield with a variety of neutral, slightly electron-rich, and slightly electron-poor aryl iodides, whereas moderate yields are obtained with aryl iodides containing strongly electron-withdrawing substituents.
http://www.bassdrive.com/BassDrive.m3u <-- 24/7 d'n'b - Music Beyond
(Chief Bee)
10-06-03 06:17
No 462965
Nifty, buy does it really offer any significant advantages over Friedel-Crafts acylation (using Polyphosphoric acid and GAA)?
(pHantasticant)
10-06-03 06:29
No 462966
Not really, but it's just another possibility...
http://www.bassdrive.com/BassDrive.m3u <-- 24/7 d'n'b - Music Beyond