(Chief Bee)
10-18-03 00:43
No 465288
(Rated as: excellent)
Regioselective demethylation of 2,6-dimethoxybenzaldehydes with magnesium iodide etherate
S. Yamaguchi, M. Nedachi, H. Yokoyama, Y. Hirai
Tetrahedron Letters 40, 7363-7365 (1999)
DOI:10.1016/S0040-4039(99)01411-2
Abstract
Demethylation of asymmetrically substituted 2,6-dimethoxybenzaldehydes with magnesium iodide etherate regioselectively single 6-methoxysalicylaldehydes derived from the more unstable chelation.
Magnesium iodide etherate is a mild reagent for the demethylation on a phenolic O-methyl group especially at the position ortho to the carbonyl group.1 In our previous paper,2 demethylation of 5-acetyl-4,6-dimethoxy-2-isopropenyl-2,3
Demethylation of two symmetric 2,6-dimethoxybenzaldehydes (1a,b) and four asymmetric 2,6-dimethoxybenzaldehydes (1c-e) with magnesium iodide etherate effectively gave single 6-methoxysalicylaldehydes (2a-e), these results are summarized in Table 1. Interestingly, in NOE measurements of the products, irradiation on the methoxy signal caused some increases (+13% in 2c, +7% in 2d, +13% in 2e) in the aromatic proton signals, and these showed the regioselective demethylation on the more hindered O-methyl groups.
Demethylation of asymmetric 3-chloro-2,6-dimethoxy-5-methylbenzaldeh
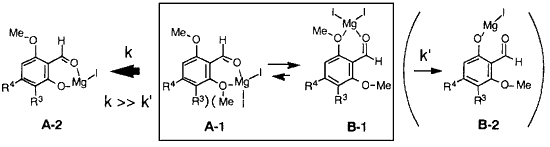
This regioselectivity might be explained as follows. Each 2,6-dimethoxybenzaldehyde can form an equilibrium of two chelation isomers, unstable A-1 and stable B-1. In the subsequent thermal elimination of methyl iodide (might be a rate-determining step), the elimination may be kinetically more favored from the unstable isomer A-1 than from the stable B-1, via the lower activation. In any case, the demethylation from the isomer, destabilized by steric or electrostatic repulsion, might be kinetically favored.
Table 1:
Demethylation of 2,6-dimethoxybenzaldehydes (1) with magnesium iodide etherate5
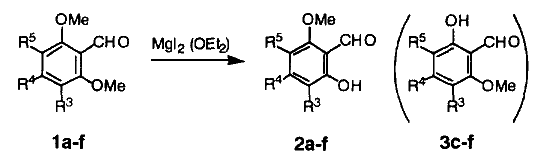
| Substrate | R3 | R4 | R5 | Reaction Time | Product | Yield |
| 1a | H | Me | H | 1.5h | 2a6 | 70% |
| 1b | H | C5H11 | H | 1.5h | 2b7 | 63% |
| 1c | Me | H | H | 4.5h | 2c4 | 84% |
| 1d | Cl | H | H | 3.5h | 2d | 94% |
| 1e | Cl | Me | H | 4.5h | 2e6 | 68% |
| 1f | Cl | H | Me | 3.0h | 2f | 90% |
References
[1] Yamaguchi, S.; Sugiura, K.; Fukuoka, R.; Okazaki, K.; Takeuchi, M.; Kawase, Y. Bull. Chem. Soc. Jpn. 57, 3607 (1984)
[2] Yamaguchi, S.; Takai, M.; Hanazome, l.; Okada, Y.; Kawase, Y. Bull. Chem. Soc. Jpn. 60, 3603 (1987)
[3] Compounds 2g and 3g were prepared by chlorination of corresponding salicylaldehydes with sulfuryl chloride, and these details will be reported soon in our synthetic study on mycochromenic acid.
[4] Details will be reported soon in our synthetic study on mycochromenic acid.
[5] General procedure for demethylation: To a benzene solution of magnesium iodide etherate, prepared from magnesium metal (102 mg, 3.99 mmol), iodine (936 mg, 3.62 mmol), ether (6 ml), and benzene (10 ml), was added a solution of 2,6-dimethoxybenzaldehyde (3 mmol) in benzene (30 ml); the mixture was refluxed, treated with 10% hydrochloric acid, and extracted with benzene, and the product was purified on a silica gel column.
[6] Details will be reported soon in our synthetic study on cannabiorcichromenic acid.
[7] Yamaguchi, S.; Shouji, N.; Kuroda, K. Bull. Chem. Soc. Jpn. 68, 305 (1995)