(Chief Bee)
05-23-04 13:05
No 509078
(Rated as: excellent)
Below I present a variety of methods for the preparation of ketones from halohydrins (principally bromohydrins), as well as the preparation of compounds of the latter class from alkenes.
As can be seen in the examples below, it is a very straightforward procedure to turn isosafrole to its bromohydrin [1-(3,4-methylenedioxyphenyl)-2-bromo-1-
However, if one's goal is to prepare MDP2P, the bromohydrin with the correct configuration [1-(3,4-methylenedioxyphenyl)-3-bromo-2-
With the bromohydrin in hand, it is transformed in high yields to the corresponding ketone with any of the procedures below, using either a palladium complex (a bit expensive, but is used in a catalytic amount), a cobolt complex (cheaper, but needs to be used in an equimolar amount) and finally with the least expensive method of all - an UV lamp and catalytic p-toluenesulfonic acid.
The Action of Bromine Water on Certain Olefinic Hydrocarbons and Ethers
John Read and William Galloway Reid
J. Chem. Soc. 1487-1493 (1928) (../rhodium/pdf /bromohydrins
It has become evident that wide use may be made of chlorine water and bromine water as reagents for the addition of hypochlorous and hypobromous acids to olefinic substances. The present paper describes the application of bromine water in this way to styrene and a number of related aromatic ethers.
Since the bromohydrins are not readily affected by hot water, it has been possible to adduce four further examples in which the yield of bromohydrin is increased by raising the temperature during the reaction with bromine water. Under the conditions adopted, the percentage yields of bromohydrin obtained at room temperature and at about 90°C, respectively, were as follows: styrene 77.9/96.7; anethole 47.9/76.1; safrole 65.2/69.2; isosafrole 67.0/72.8. It thus appears to be a general rule that when the halogenohydrin is stable towards hot water the ratio halogenohydrin/dihalogenide increases with rise of temperature. The yield of halogenohydrin is increased also by dilution.
A Palladium Catalyzed Conversion of Halohydrins to Ketones
Jiro Tsuji, Hideo Nagashima, and Koji Sato
Tetrahedron Letters 23(30), 3085-3088 (1982) (../rhodium/pdf /halohydrin2k
Summary
Pd(OAc)2 combined with P(o-Tol)3 catalyzes the conversion of halohydrins to ketones in the presence of K2CO3. Various halohydrins, which are easily available from olefins, can be converted to ketones in high yields.
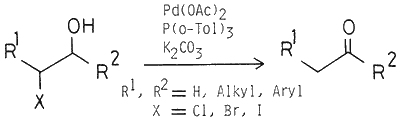
Conversion of halohydrins to ketones promoted by ethylmagnesium halide or silver nitrate has been reported by House1. However, this method is not satisfactory due to poor yields of ketones. Halohydrins are easily available from olefins, and in some cases, regioselective preparation is possible2. Thus, the preparation of halohydrins from olefins and the subsequent conversion of halohydrins to ketones are expected to be a useful preparative method of ketones from olefins.
In this paper, we wish to report the palladium catalyzed conversion of halohydrins to ketones. By this procedure, a useful preparative method of ketones from olefins is established. As described in our previous paper3, reaction of 1-hepten-3-ol with BrCCl3 or CCl4 catalyzed by Pd(OAc)2 combined with tri-o-tolylphosphine (P(o-Tol)3) affords 1,1,1-trichloro-4-octanone (8) in the presence of K2CO3 at 110°C.
When this reaction was carried out at 40°C otherwise under the same conditions, the halohydrins 1a and 16 were formed as the main product. This result suggests that 1a and 1b are intermediates to form 8. We found that treatment of 1a and 1b with a catalytic amount of Pd(OAc)2 combined with P(o-Tol)3 in the presence of K2CO3 at 110°C afforded 8 in 87 and 57% yields, respectively. In the absence of palladium, only a trace amount of the ketone was formed. In this reaction, choice of ligand and solvent is important. P(o-Tol)3 as the ligand gave higher yields of 8 than PPh3, tricyclohexylphosphine, and 1,2-bis(diphenylphosphino)ethane. Use of polar solvents such as t-butyl alcohol, acetonitrile, and THF decreased the yield of 8. In all cases, a small amount of 1-(2,2,2-trichloroethyl)-2-butyloxirane was detected. This epoxide formation is considered to take place with the aid of K2CO3.
As shown in the Table, other halohydrins were also converted to the corresponding ketones by using Pd(OAc)2/P(o-Tol)3 catalyst in the presence of K2CO3 in benzene or toluene at 80-110°C. ln the reaction of bromohydrins, 2, 3, and 4, which possess secondary bromide, and 5, which possesses tertiary bromide, the corresponding ketones were obtained in reasonable yields. Only trace amounts of the corresponding epoxides were detected. On the other hand, halohydrins having primary halide such as 6 and 7 were converted to the corresponding ketones in lower than 50% yields. In these cases, considerable amounts of the corresponding epoxides were also formed.
In a typical example, in a flask fitted with a reflux condenser were placed Pd(OAc)2 (2 mg, 9.01 mmol), P(o-Tol)3 (6 mg, 0.02 mmol), and K2CO3 (138 mg, 1 mmol) and the atmosphere was replaced by argon. The bromohydrin 2 (215 mg, 1 mmol) dissolved in benzene was added, and the suspension was refluxed for 5 h under argon atmosphere. The potassium salts were filtered off, and the filtrate was concentrated in vacuo. The residue was purified by column chromatography (silica-gel, hexane-ether) to give propiophenone (9) in 87% yield.
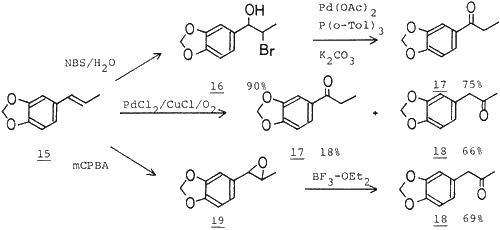
As noted above, preparation of halohydrins from olefins and subsequent conversion of the halohydrins to ketones provide a preparative method of ketones from olefins. As a typical example, we carried out the reaction of isosafrole (15). Treatment of 15 with N-bromosuccinimide in aqueous DMSO afforded the bromohydrin 16 in quantitative yield as a sole product. No regioisomeric bromohydrin was detected. Then, 16 was treated with Pd(OAc)2 (5 mol%)/P(o-Tol)3 (10 mol%) catalyst in the presence of K2CO3 (100 mol%) in benzene at 80°C for 3 h to give the ethyl ketone 17 in 75% yield. It is worthwhile to point out that by other preparative methods of ketones from olefins, such as palladium mediated oxidation of olefinic bond4, and epoxidation of olefins and subsequent Lewis acid catalyzed isomerization of the epoxides to ketones5, isosafrole (15) is converted to the methyl ketone 18 with high selectivity. These results imply that the preparative method of ketones from olefins via bromohydrins is useful, especially when the bromohydrins are formed from olefins with high regioselectivity.
References
[1] H. O. House, J. Am. Chem. Soc., 77, 5083 (1955)
[2] D. R. Dalton, V. P. Dutta, and D C. Jones, J. Am. Chem. Soc., 90, 5498 (1968) (../rhodium/pdf /halohydrins.
[3] H. Nagashima, K. Sato, and J. Tsuji, Chem. Lett., 1605 (1981)
[4] J. Tsuji, I. Shimizu, and K. Yamamoto, Tetrahedron Lett., 2975 (1976)
[5] For example, D. R. Reif and H. O. House, Org. Syn., Coll. Vol. 4, 375 (1963) (http://www.orgsyn.org/orgsyn/prep.asp?p
Palladium-Catalysed Oxidation of Alcohols with Carbon Tetrachloride [...] and Conversion of Halohydrins to Ketones
Hideo Nagashima, Koji Sato and Jiro Tsuji
Tetrahedron 41(23), 5645-5651 (1985) (../rhodium/pdf /halohydrin2k
Abstract
Pd salts catalyse oxidation of alcohols with CCl4 in the presence of K2CO3. Primary alcohols are oxidised to esters, and secondary alcohols to ketones. CCl4 is converted to CHCl3. The reaction of allylic alcohols bearing a terminal olefinic bond with CCl4 or BrCCl3 in the presence of palladium catalyst at 110°C affords 4,4,4-trichloro ketones. At 40°C, simple adducts of CCl4 or BrCCl3 having a halohydrin structure are obtained, which are converted to the corresponding trichloro ketones by the catalysis of palladium. Various halohydrins are converted to ketones by Pd catalysis.
Oxidation of isosafrole
Method A (via bromohydrin)
In a pyrex tube fitted with a screw cap were placed Pd(OAc)2 (11.2 mg, 0.05 mmol), P(o-Tol)3 (30.4 mg, 0.1 mmol) and K2CO3 (138 mg, 1 mmol) under argon. The bromohydrin (259 mg, 1 mmol) dissolved in benzene (1 ml) was added, and then the mixture was heated at 100°C for 5 hr. After the work-up as above, purification of the residue by column chromatography (silica gel, hexane-ether) afforded the desired methylenedioxypropiophenone.
Method B (via epoxide)
To a soln of isosafrole (1.62 g, 10 mmol) in CH2Cl2 (10 ml) was added mCPBA (3.24 g, 15 mmol) dissolved in CH2Cl2 (10 ml). The mixture was stirred at 0°C for 30 min. Usual work-up and chromatographic purification (silica gel, hexane-ether) afforded the epoxide (190 mg) in 11% yield. The epoxide (190 mg, 1.07 mmol) was treated with BF3·Et2O (1 ml) in ether at room temp for 20 min. The soln was quenched with NaHCO3 aq, and the ethereal layer was washed with brine and dried over MgSO4. After removal of the solvent, the residue was purified by column chromatography (silica gel, hexane-ether) to give methylenedioxyphenylacetone in 69% yield (132 mg).
Method C (Wacker oxidation)
In a flask fitted with a balloon filled with O2 were placed PdCl2 (35.5 mg, 0.2 mmol) and CuCl (79.2 mg, 0.8 mmol) in aqueous DMF (DMF-H2O 7:1, 2 ml). The suspension was stirred under O2 at room temp for 2 hr. Then, isosafrole (324.4 mg, 2 mmol) dissolved in the aqueous DMF (2 ml) was introduced. and the mixture was heated at 50°C for 10 hr. Usual work-up and purification by column chromatography (silica gel. hexane-ether) afforded methylenedioxypropiophenone (236 mg, 66%) and methylenedioxyphenylacetone (65 mg, 18%).
Photochemical Conversion of Bromohydrins to Ketones
Olivier Piva
Tetrahedron Letters 33(18), 2459-2460 (1992) (../rhodium /halohy
Abstract
The direct conversion of halohydrins to ketones can be achieved by irradiation in benzene or toluene in the presence of small amounts of p-toluenesulfonic acid. A two step conversion of terminal alkenes to methylketones is thus achieved with good yields and inexpensive reagents.
____ ___ __ _
Reaction of Bromohydrins with Chlorotris(triphenylphosphine)Cobalt(I)
Den-ichi Momose and Yasuii Yamada
Tetrahedron Letters, Vol.24, No.26, pp 2669-2672 (1983) (../rhodium/pdf /halohydrin2k
Summary
Bromohydrins were converted into ketones in high yields by the reaction with chlorotris(triphenylphosphine)cobalt(I) in the presence of amine or olefin. A probable pathway for the formation of ketones from bromohydrins was also described.
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
05-26-04 16:18
No 509802
(Rated as: excellent)
Here are more related reading about reaction sequences going from alkene to bromohydrin to benzyl ketones (in this case 2-indanone, but it is very closely related to phenyl-2-propanone). Included is a procedure to turn a propenylbenzene bromohydrin to an epoxide (for rearrangement to a phenylacetone) so that we aren't limited to only using allylbenzenes.
The Action of Bromine Water on Indene
John Read and Eric Hurst
J. Chem. Soc. 121, 2550-2554 (1922) (../rhodium/pdf /indene.bromo
____ ___ __ _
Über das 2-Indanon
Wilhelm Treibs & Werner Schroth
Ann. Chem. 639, 204-213 (1961) (../rhodium/pdf /2-indanone.b
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
05-31-04 23:00
No 510665
(Rated as: good read)
Direct Conversion of Bromohydrins to Ketones by a Free Radical Elimination of Hydrogen Bromide
Darko Dolenc and Maja Harej
J. Org. Chem. 67, 312-313 (2002) (../rhodium/pdf /bromohydrin2
Abstract
Secondary β-bromo alcohols can be transformed directly to ketones in very good yields by a free radical process. Tertiary β-bromo alcohols do not react while the primary ones are transformed to aldehydes in lower yields. The reaction involves an abstraction of a hydrogen atom R to an OH group, followed by elimination of the bromine atom and subsequent tautomerization of an enol to a ketone.
The Hive - Clandestine Chemists Without Borders
(Hive Addict)
06-28-04 10:19
No 516025
However, if one's goal is to prepare MDP2P, the bromohydrin with the correct configuration [1-(3,4-methylenedioxyphenyl)-3-bromo-2-
Ok, I'm confused... You said above that in order to obtain the phenylacetone instead of the propiophenone, one needs to start from the allylbenzene and not propenylbenzene and linked the page suggesting a method for MDP2P... The link says isosafrole dibromide.....?
(Chief Bee)
06-29-04 09:50
No 516314
Well, the topic of this thread was the one-step conversion of bromohydrins to ketones. Only safrole bromohydrin will convert to MDP2P like this, isosafrole bromohydrin would instead give 3,4-methylenedioxypropiophenone. The document you linked offers an alternative route from isosafrole bromohydrin to MDP2P, using a two-step reaction via isosafrole epoxide.
Above I inferred that the same dibromination and debromination-hydroxylation used with isosafrole to give isosafrole bromohydrin could be used with safrole to yield safrole bromohydrin.
All roads lead to Rome (or MDP2P in this case)...
The Hive - Clandestine Chemists Without Borders