(Hive Bee)
06-22-04 23:37
No 514831
(Rated as: excellent)
Here are two articles on the reduction of the carbon double bond in nitrostyrenes with tributyltin hydride as the reducing agent. For related methods see Post 352950 (Barium: "Get that double bond without borohydride", Novel Discourse).
Before trying this method please inform yourself about the really highly toxic and dangerous chemicals:
Tributyltin hydride
H2F2, hydrogen fluoride
Reduction of alpha-beta-unsaturated nitrocompounds with tributyltin hydride
J.M. Aizpurua, M. Oiarbide, C. Palomo
Tet. Lett., 1987, 28(44), 5365-5366

Abstract: Nitroalkenes upon treatment with tributyl tin hydride in the absence of any catalyst provide a new method for the reduction of alpha,beta-unsaturated nitrocompounds. Oxidative cleavage of the intermediate stannyl nitronates yielded carbonyl compounds in good yields.
In the preceding paper we described a convenient method for the preparation of carbonyl compounds via Nef reaction on trialkylsilyl nitronates. In this communication we report our preliminary results on the reduction of nitroalkanes by means of tributyltin hydride2.
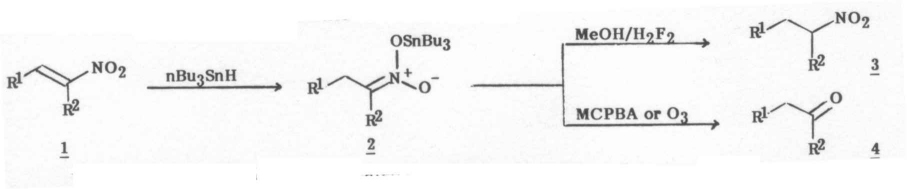
The tin hydride reduction of unsatureated carbon-carbon bonds is well documented3, however to our knowledge no reports have been described concerning the reduction of alpha,beta-unsaturated nitrocompounds promoted by tin hydrides3, 4. Our new finding is that treatment of 1 with tributyltin hydride (1.2 equiv) in methylene chlrodie at room temperature for 20-24 h followed by work-up3b afforded the corresponding nitroalkane 3 in high yield. Some examples are given in the Table to illustrate this method. For primary nitroalkanes work-up involved treatment of the reaction mixtures with methanol (2 ml), evaporation of the solvent and idlution with methanol followed by addtion of hydrofluoric acid (2N in MeOH). The precipitate tin compounds were filtered off and the nitrocompound was seperated by column chromatography on silical gel and purified by Kugelrohr distillation. Under these conditions alpha-substituted tin nitronates afforded the expected secondary nitroalkanes together with the corresponding ketones. We observed that unsubstituted tin nitronates are very sensitive to moisture whereas the alpha-substituted ones are stable under the above work-up conditions and therefore a concomitant Nef reaction also takes place (entries b and h). Of the solvents examined, methylene chloride, benzene and methanol were the most effective; acetonitrile, dimethoxyethane and tetrahydrofuran were found unsuitalbe for carry out reduction of nitroalkenes by means of tributyltin hydride. In benzene the reaction was extremely slow but it can be accelerated by the addition of methanol. Also, we have found that tributyltin trifluoromethanesulfonate5 notably enhances tin hydride reduction of nitroalkenes in methylene chlrodie or benzene as solvents. Also, it is worth of note that under the conditions reported here no dimeric products were formed during tin hydride reduction of nitroalkenes6.
Further applications of the present procedure can be illustrated in the high yield formation of ketones from stannyl nitronates in a single pot operation. For example when a beta-methyl-beta-nitrostyrene (5 equiv.) in methylene chloride (5 ml) was subjected to treatment with tributyltin hydride (5.2 equiv.) overnight fllowed by addition of MCPBA (5.2 equiv.) in methylene chloride (10 ml) the corresponding ketone was isolated in high yield. Attemps to prepare aldehydes from beta-nitrostyrenes 1 (R2 = H) by MCPBA oxidation of the corresponding stannyl nitronates failed to give good results. This fact may be adscrbied to an easy destannylation of the tin nitronate as we have mentioned above. However, we have found that treatment of tin nitronates with ozone afforded good yields in the expected aldehydes. For example when nitrostyrene was treated with tributyltin hydride under nitrogen atmosphere overnight and then the resulting mixture was subjected to reductive ozonolysis, phenylacetaldehyde was obtained in 72% isolated yield together with 1-phenyl-2-nitroethane in 10% isolated yield.
In conclusion we have found new applications of tributyltin hydride in organic synthesis. Importantly, the method allows reduction of nitroalkenes under midl reaction conditions without need any catalyst and therefore it could be used in selective reductions. Further investigations to explore the scope and limitations of the method as well as "in situ" utilization of the intermediate stannyl nitronate are in progress in our laboratory.
Table Reaction of nitroalkenes 1 with nBu3SnH.
| Entry | Compound 1a: R1 | Compound 1a: R2 | Product 3b: Yield, %c | Product 3a: b.p. °C/torrd or m.p. °C | Product 4b: Yield, %c | Product 4a: b.p. °C/torrd or m.p. °C |
| a | C6H5 | H | 90 | 92/0.05 | 72e | 200/760 |
| b | C6H5 | CH3 | 50f | 97 | 115/20 | |
| c | 4-CH3C6H4 | H | 80g | 135/0.06 | 70e | 105/0.05 |
| d | 4-CH3C6H4 | CH3 | --- | --- | 95 | 150/20 |
| e | 3-O2NC6H4 | H | 80 | 59 | --- | --- |
| f | 4-CH3C6H4 | CH3 | --- | --- | 99 | 120/17 |
| g | 4-ClC6H4 | H | 99 | 135/1.0 | --- | --- |
| h | 4-NCC6H4 | CH3 | 50h | 150/0.5 | 95 | 72-73 |
| i | CH2CH3 | CH3 | --- | --- | 72 | 105/760 |
a These compounds were prepared by the method described by J. Bourguignon, G. Le Nard, G. Queguiner, Can. J. Chem., 63, 2354 (1985)
b All products exhibited physical and spectral characteristics in accordance with the assigned structures
c Isolated yields
d Reported boiling points are those observed during distillation with a Kugelrohr apparatus and are uncorrected
e By reductive ozonolysis of the corresponding stannyl nitronates; 10% of the nitroalkane was also isolated
f 50% of the ketone was isolated
g 5% of the corresponding oxime was isolated
h 45% of the ketone was isolated
References and notes
1. Reagents and Synthetic Methods 66. For part 65 see the preceding paper. The present work has been supported by Departamento de Investigacion del Gobierno Vasco (Eusko Jaurlaitzako Hezkuntza Saila). A grant from Eusko Jaurlaritza to M.O. is gratefully acknowlegded.
2. a) M. Fieser and L. Fieser "Reagents for Organic Synthesis", Wiley, New York, Vol II (1984) and references cited; b) G.S. Bristow, Aldrichimica Acta, 17, 75 (1984).
3. a) H.G. Kuivila, Synthesis, 499 (1970); b) For a recent review on tin compounds in organic synthesis see: M. Pereyre, J.P. Quintard, A. Rahm, "Tin in organic synthesis", Butterworths, London, 1987 p. 122 and references cited.
4. For a recent review on reduction see: M. Hudlicky "Reduction in Organic Chemistry", Ellis Horwood Limited, John Wiley, New York, 1984
5. Y.T. Xian, P. Four, F. Guibe, G. Balavoine, Nouv. J. Chim., 8, 611 (1984).
6. a). S Schechter, D.E. Ley, E.B. Roberson Jr., J. Am. Chem. Soc., 78, 4984 (1956); b) A.G.M. Barret, G.G. Grabosky Chem. Rev., 86, 751 (1986).
(Received in UK 30 June 1987)
Tributyltin Hydride Addition to Nitroalkenes: A Convenient Procedure for the Conversion of Nitroalkenes into Nitroalkanes and Carbonyl Compounds
Claudio Palomo, Jesus M. Aizpurua, Fernando P. Cossio, Jesus M. Garcia, M. Concepcion Lopez, and Mikel Oiarbide
J. Org. Chem., 1990, 55(7), 2070-2078

Abstract: A new procedure for the reduction of nitroalkenes by using n-tributyltin hydride as reducing agent is described. The reaction proceeds under almost neutral conditions and works well even in the presence of other reduceable functionalities. Hydrolysis and Nef reaction of the resulting nitronates furnished nitroalkanes and carbonyl compounds respectively in high yields. Application of this methodology to the preparation of beta-3-lactam building blocks is also made.
[...]
Reduction of beta-Nitrostyrenes 5 to Nitroalkanes 7. General Procedure.
To a solution of the corresponding nitrostyrene 5 (R, = Ar, R2 = R3 = H) (3 mmol) in methylene chloride (7.5 mL) was added tributyltin hydride (0.95 mL, 3.6 mmol), and the resulting mixture was stirred at room temperature. The conversion of the reaction was monitored by 1H NMR spectroscopy from an aliquot of the reaction mixture. When the conversion was total the solvent was evaporated under reduced pressure. The resulting oil was dissolved in methanol and treated with a solution of H2F2 in methanol. The resulting precipitate tin compounds were filtered off and the residue was subjected to column chromatography to afford the corresponding nitroalkane, which was purified by distillation or crystallization. All compounds exhibited physical an spectral characteristics in accordance with the assigned structures.
[...]
Preparation of Primary Nitroalkanes. General Procedure.
To a solution of the corresponding nitroalkene (6 mmol) in methylene chloride (20 mL) and methanol (2 mL) was added tributyltin hydride (1.85 mL, 7.2 mmol), and the mixture was stirred at room temperature for 24 h. Evaporation of the solvent gave an oil, which was triturated with ethanol and filtered off to give the corresponding nitroalkane. An analytical sample was obtained by crystallization from ethanol.
Preparation of Secondary Nitroalkanes. General Procedure.
To a solution of the corresponding alpha-substituted nitroalkene (6 mmol) in methylene chloride (20 mL) was added tributyltin hydride (1.9 mL, 7.2 mmol), and the mixture was stirred at room temperature for 24 h. Evaporation of the solvent gave an oil, which was dissolved in methanol (15 mL). To this solution was added H2O (2 mL), and two phases appeared. To this mixture was added glacial acetic acid (1.2 mL), and the resulting solution was stirred at room temperature for 30 min from which a white precipitate appeared. Stirring was continued for 5 h at the same temperature and the precipitate was filtered off to give the nitroalkane, which was purified by crystallization from ethanol.
[...]
Preparation of Ketones. General Procedure.
The corresponding methylene chloride solution of the alpha-substituted tin nitronate prepared as above was diluted with the same solvent (30 mL) and the solution was cooled to -78 °C. A stream of ozone was passed through the reaction mixture until a pale blue coloration was observed and then the solution was purged with nitrogen. A solution of Me2S (4 mL) in methylene chloride (10 mL) was added dropwise at -78 °C. When the addition was completed, the bath was removed and the solution was stirred until it reached room temperature. The reaction mixture was washed with H2O (25 mL) and NaCl (3 X 30 mL, saturated solution). The organic layer was separated and dried (MgSO4). Evaporation of the solvent gave a residue, which was diluted with methanol and treated with a solution of H2F2 in methanol, and the resulting precipitate tin compounds were filtered off and the residue was subjected to column chromatography. In other cases the residue was directly crystallized from ethanol to give the corresponding ketone.
The tendency is to push it as far as you can