07-26-04 12:24
No 521839
Does anyone know of a synth route to phenylpiperazines that doesn't involve 2-(bis)dichloroethylamine, or like compunds? I was thinking of bromo- or iodo benzenes + piperazine + base, with inert substituents elsewhere on the benzene ring.
(Hive Bee)
07-26-04 15:56
No 521871
Bromo and iodobenzene aren't like their alkyl derivs. The aromatic nucleus means that they wont directly substitute into amines the way, say iodoethane will (although 2,4-dinito-iodobenzene will due to effects of NO2 groups, but I don't think the end result is what you would be looking for). There will be other synthetic routes, but this isnt one of them
That is right, the Mascara Snake: Fast and bulbous
(Newbee)
07-26-04 21:32
No 521924
Physical characterization of the new bis(N-phenylpiperazines). Journal of Pharmacy (University of Karachi) (1985), 3(2), 89-94.
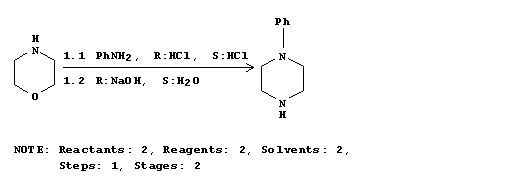
This has details of the formation of phenylpiperazines using potentially un/substituted anilines and morpholine to give the phenyl piperazine using simple lab reagents. I'll try to get access to this article and upload it but if someone else beats me to finding it and accessing it... thats fantastic.
Cheers
(«
 »)
»)07-27-04 02:16
No 521959
I had a write-up for you and everything detailing the chlorination of benzenoids via TCICA, and all the relevant data you would need to synthesize the desired phenylpiperazines from said chlorobenzenes and whatever amine you saw fit to employ in the reaction.
I'll have to get the info to you later, likely sometime during the week.
Sorry, for now.
07-28-04 00:19
(Rated as: UTF PM Function)