09-23-04 17:55
No 532855
There has been a lot of interest on this forum lately in heterocyclic analogues of MDMA and other psychedelic amphetamines. I would just like to touch on a few areas of interest that I have been working in. The research of Dr. Nichols and his laboratory has opened the door for many possible psychedelics with novel structures. In one of his papers he describes the activity of benzofuran, indane, and tetralin derivatives of MDA. The indane derivative, IAP, has received much attention on this forum; the other's not as much. The benzofuran derivatives seem to be very similar in potency to MDA, while the deoxygenated derivative, IAP, is actually of increased potency. He also describes that the aromatic benzofuran analogues are of increased potency relative to the saturated dihydrobenzofuran analogues. Since naphthalene is nothing more than an aromatised tetralin, this got me thinking about possible compounds that could be derived from this substance which can be obtained in great purity even from grocery stores (moth balls). The main problem that I see here is that the alpha position of naphthalene is the most activated position almost any attemps at substitution of this nucleus result in substitution in this position. This places the additional ring in a not so favorable position (technically the 2 and 3 position) when it comes to creating a compound of potency. Here is my first attempt to create an active compound from naphthalene:
Naphthalene was brominated utilizing the H2O2/HBr system yielding a yellow solid that is undoubtedly alpha-bromo-naphthalene. This brominated product was then chloromethylated with HCl/CH2O in HOAc. The chloromethylation proceded smoothly but I am unsure as to the product of this reaction. I referenced my handy org. chemistry textbook which states that deactivating substituents in the alpha position leads to subsequent substitution in the alpha' position, which is not desirable for me. However, the examples shown in the book only display sulfonated and nitrated alpha-naphthalenes which are both meta-directing, so I can see how this would lead to substitution in the alpha' position. My hope is that the para-directing effect of the bromine atom lead to chloromethylation in the 4 position, and will lead to the following end product;
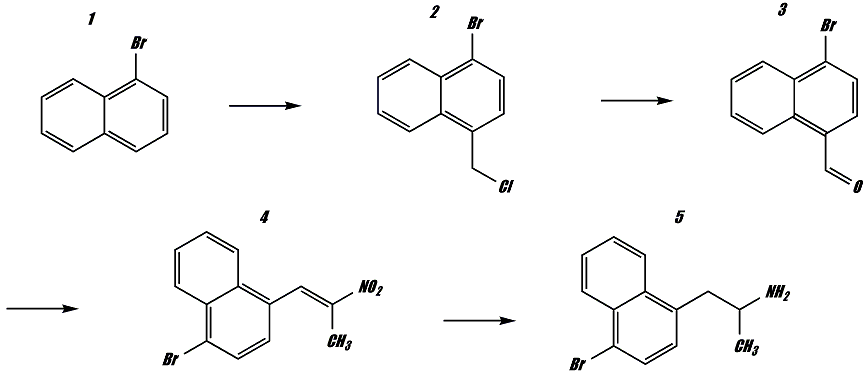
Yes, this still places the additional ring in the second and third positions, but it also places a bromine atom in the 4 position which should increase potency. My only hope is that the neurotoxic effects of the monobrominated p-bromoamphetamine will not be present in this compound.
The direct MDA derivative will be a little harder to synthesize but here is the only potential pathway I know of:
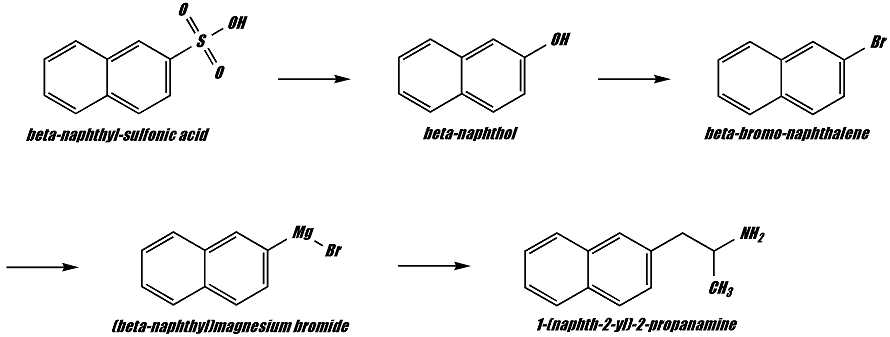
According to my textbook references, sulfonation of naphthalene at high temperature (<150deg C) leads to beta-sulfonation of the naphthalene nucleus. Hydrolysis of this sulfonated product will lead to beta-naphthol. Reaction of this naphthol with phosphorus trihalide will lead to a beta-halonaphthalene. From here grignard formation will take place which can lead to the desired product by a variety of potential routes:
Another idea that seems to have been neglected is the possibility of an indenyl-amphetamine analogous to IAP. Indene is less availably however and the only synthesis I know of is from the dehydrogenation of o-ethyltoluene. Again, there is the possibility of even higher potency here than IAP. Questions or comments about any of this material are welcome, I have references for everything stated which are available upon request..
- phenethylman -
(Hive Bee)
09-23-04 23:11
No 532896
In terms of their action on serotonergic systems, yes IAP is indeed more active on a weight for weight basis, but it is nowhere near as potent in terms of the dopaminergic systems. It appears that in order to produce MDA like effects, the compound needs to be active with both serotonin and dopamine systems. (I have tried a combination of IAP and amphetamine together, each having their main activity w.r.t. serotonin and dopamine respectivly, and it produces a reasonably close approximation to MDA). Drugs such as 3-methoxy-4-methylamphetamine and 5-methoxy-6-methyl-2-aminoindane (MMAI) are also more potent than MDA when it comes to the serotonergic systems, but as they have very little effect w.r.t. dopamine, they are unlike MDA/MDMA.
N-methylation of any of the compounds above may give a compound more like MDA, but I've been unable to find any papers that refer to the activity of N-methyl IAP (or any of the others), so until somebody actually synths and tastes them, we're stll in the dark
That is right, the Mascara Snake: Fast and bulbous
(Moderator)
09-23-04 23:15
No 532897
Reaction of this naphthol with phosphorus trihalide will lead to a beta-halonaphthalene.
I doubt that...
Why don't you use the commercially available 2-naphtaldehyde? The 2-naphtylethylamines are an interesting class of compounds. Glennon once stated in a review article that the 5-HT2A receptor affinity of 2-naphtylisopropylamine is more than two orders o magnitude that of amphetamine. But if you are interested in MDMA-like effects I would test the N-methyl compound.
(Hive Bee)
09-23-04 23:32
No 532900
Very nice to see a bee actually working on the naphthaleneethylamines. Actually on anything that is not already “known and loved” by someone else.
I don’t fully agree on your logic of extending the empathogens SAR, but I’m almost sure the target compounds will bee psychoactive in some way. I prefer to look at naphthalene as an indole isostere rather than benzene-like.
Somehow I was convinced I read about the 1- and 2-isopropylaminonaphthalenes on The Hive, but could find nothing with TFSE, except Post 122808 (dormouse: "Replacing Benzene with Naphthalene -Nemesis", Serious Chemistry). False memory I guess or maybe Lilly can tell the reference?
Phenylethyl, could you please post the method you used for the chloromethylation of bromonaphtalene. It seams a little contradictory that plain AcOH/HCl would already be enough acidic when most methods use some additional dehydrative acid for the chloromethylation of naphthalene*. And bromonaphtalene is even slightly deactivated. Actually, I once tried to chloromethylate 2-MeO-naphtalene in AcOH/HCl, thinking it would be easy, so I heated it only to 70°C and it did not react (BTW: otherwise beta-methoxynaphtalene gets chloromethylated at the position 6**).
* http://www.orgsyn.org/orgsyn/prep.asp?pr
** http://voh.chem.ucla.edu/vohtar/spring01
Lilienthal, I think phenylethyl may be right with PBr3 + beta-naphthol because naphtoles are known to be intermediary between ketones and phenols with their properties (they even react with bisulphites, though the equilibrium is much more to the left).
Questions or comments about any of this material are welcome, I have references for everything stated which are available upon request.
It’s easier to comment if you let us check all the data that you used. Don’t you think so? References are always useful, so please add them to the post.
Good luck with the next steps.
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Chief Bee)
09-23-04 23:41
No 532902
[alpha-bromo-naphthalene] was then chloromethylated with HCl/CH2O in HOAc. The chloromethylation proceded smoothly but I am unsure as to the product of this reaction. [...] However, the examples shown in the book only display sulfonated and nitrated alpha-naphthalenes which are both meta-directing, so I can see how this would lead to substitution in the alpha' position. My hope is that the para-directing effect of the bromine atom lead to chloromethylation in the 4 position...
I am unsure of that. Bromine is deactivating in addition to its o,p-directing property, so I would rather believe that you would get substitution on the other ring, rather than para to the Br. I'd think that bromination of α-chloromethyl-naphtalene would be a much better way of arriving at the desired 1-bromo-4-chloromethyl-naphthalene.
There is only one mention of that compound in Beilstein, and that's M.I. Terekhova, et al.; J.Org.Chem.USSR (Engl.Transl.), 23, 361-369 (1987); Zh.Org.Khim., 23(2), 403-412 (1987)
The final amine and the corresponding naphtyl-2-propanone oxime is mentioned in the Tetrahedron article posted in Post 454846 (Rhodium: "Here are a few of the references located", Methods Discourse)
The Hive - Clandestine Chemists Without Borders
(Newbee)
09-24-04 16:33
No 532985
fastandbulbous; The N-methyl analogues of both IAP and NAP (naphthyl-amino-propane, I just made that up for lack of a better term.) interest me as well. What interests me even more is the alpha-ethyl and N-methyl-alpha-ethyl analogues. There is no doubt that MBDB/BDB are fascinating compounds and I would expect more of the same. There's no reason not to synthesize these when synthesis is no more difficult; merely substituting 1-nitropropane for nitroethane in the condensation.
Lilienthal; actually, you are right, but it was only an error of mine. It is phosphorus tetrahalide (PBr5 or PCl5) that is required for this reaction to take place. I don't have any online references for this reaction; nor have I searched for them. This is in an organic chemistry book of mine: Noller, Carl R. (Carl Robert), Chemistry of organic compounds [by] Carl R. Noller. Hah, I know this is ancient but it is a great book with tons of information on heterocyclics. And since it was written before the "drug war" hysteria set in it is quite liberal in this area. Routes to several barbituates and amphetamine are provided (no substituted phenethylamines, although mescaline is briefly mentioned.) LSD is briefly mentioned as a compound that produces a psychosis much like schizophrenia.
I would rather not just purchase the aldehyde because I am interested in the chemistry in getting to that point, and also because simply buying the aldehyde may not always be an option for me.
nicodem; okay, here is a brief overview of how I did the chloromethylation. A mixture of HOAc, an equimolar amount of formalin solution, and a catalytic amount of phosphoric acid was saturated with HCl gas. When the substrate was added under stirring the mixture was quite heterogenous so a PTC was also added. This was allowed to stir rapidly at room temperature for 24-36 hours (probably excessive, but it was the next time I could get back to the lab.) At this point the mixture had changed to a much lighter yellow color. The reaction was flooded with ice-cold dH2O and allowed to stir for 15 minutes and the product precipitated as a yellow solid which was isolated by vacuum filtration.
Formylation went like this: To a solution of water and 2-nitropropane (equimolar to the substrate) was added a saturated equimolar NaOH solution. This was allowed to stir at room temperature for 1 hour. The substrate dissolved in denatured alcohol was then added and the whole stirred on a water bath at 50-60deg C for 2 hours. It was then stripped of volatiles under vaccum to reveal a semi-solid brown residue. At this point I simply flooded the residue with cold dH2O and a white-solid precipitated out of the dark-brown reaction mix; it was isolated by vacuum filtration. The white solid smells like an aldehyde, is insoluble in water, and forms a bisulfite addition product. Yields were not the greatest but I used a huge amount of substrate since all these starting materials are so cheap. However, the question that still remains; in what position has this been formylated?!?
The problem w/refs is that most of my references are from books and I don't have any way to scan them in, of course I can still list them and will upon request. The chloromethylation was completely improvised on my part.. The reaction of 2-nitropropane/NaOH I used for formylation which parallels the sommelet reaction is on rhodium's site at: ../rhodium /halide.
rhodium; I have done this before; I just didn't like working with alpha-chloromethyl-naphthalene. It was a nasty yellow liquid and so was the 1-naphthaldehyde. Brominating the naphthalene first allowed me to work with a solid product throughout.
Here is another route to the desired aldehyde for NAP which I think I may find preferable to working with grignard's:
Following the beta-sulfonation and hydrolysis to beta-naphthol (see the first posting), the alcohol undergoes ammonlysis to form beta-naphthalamine. This product is then diazotized and reacted with the oxime of formaldehyde (see: http://www.orgsyn.org/orgsyn/orgsyn/prep
- phenethylman -
(Hive Bee)
09-26-04 02:13
No 533185
The synthesis of nabumetone [42924-53-8] might help shed some light on this subject.
Nabumetone is 4-(6'-methoxy-2'-napthyl)-butan-2-one, which you may notice is a homologue of 1-(6'-methoxy-2'-napthyl)-propan-2-one.
From the propanone, a straightforward reductive amination should give you a potential compound of interest. I would avoid using a napthalenyl carboxaldehyde as your precursor as Shulgin mentions he had no luck at all with that route. I hope this information helps you in some way, or at least provides you a workable starting point.
(Newbee)
09-26-04 17:57
No 533251
Thanks for the interesting information. Hell, if only this compound were a 3-butanone rather than a 2-butanone it would most likely lead to a most interesting alpha-ethyl phenethylamine upon reductive amination.
Of course, it is most unfortunate that the condensation they use of 2-naphthaldehyde with acetone leads to the former ketone, and no preparation of this aldehyde is provided in the journal article.
Could you please elaborate on where Shulgin mentions having no luck with naphthaldehydes? I assume you mean during the condensation w/the nitroalkane? I find this odd since he utilizes naphthaldehydes in the preparation of 2C-G-N and G-N in pihkal [http://www.erowid.org/library/books_onli
- phenethylman -
(Hive Bee)
09-29-04 07:43
No 533725
On a bit more lighthearted note (and I hope he doesn't mind), I'd discussed N-methyl IAP with Kinetic in the past, and because N-methyl IAP looks messy, I suggested MIAP as a shortened name, but he played a blinder and sugessted IMP [5-Indanyl-(2-Methylamino)Propane], and I've got to say, I like it - a very Shulginesque name.
I just hope it is a significant entactogen
That is right, the Mascara Snake: Fast and bulbous