The reaction between phenylacetic acid and acetic anhydride in the presence of pyridine was originally described by Dakin and West1 who noted that the main product was benzyl methyl ketone. King and McMillan2 have re-investigated this reaction and shown that dibenzyl ketone is also formed. They consider the reaction to be a “base-catalysed condensation reaction of two acid anhydride molecules”, and propose the above mechanism.
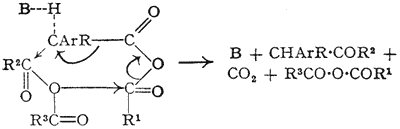
We were also engaged on this same problem, and our results are very similar. We agree that the reaction is essentially that of an acid anhydride in the presence of a base, but disagree with the mechanism proposed for these reasons. If the reaction is a condensation reaction as shown above, the essential step is the acylation of the reactive methylene group by a molecule of anhydride.
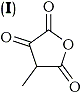
It would therefore be expected that the similarly active methylene group of phenylacetic ester would be acylated under the reaction conditions, yielding ethyl α-phenylacetoacetate. This, we find does not take place. Even the more reactive benzyl cyanide, which would be expected to yield α-acetylbenzyl cyanide, fails to react, and in each case the starting material was recovered in almost quantitative yield. Moreover, such a mechanism assumes that a β-keto-acid anhydride will readily lose carbon dioxide, although it is known3 that at least one such substance (I) is relatively stable.
We envisage a migration mechanism as the only one which satisfactorily accounts for all the facts:
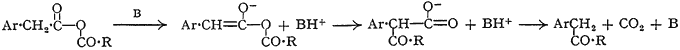
The rearrangement step finds a parallel in the O → C migration of the acyl group in acylated β-keto-esters4. It is also possible that the recently reported5 rearrangement of the enol acetates of ketones represents a less favourably activated example of the same reaction.
Experimental
Ethyl phenylacetate (45 g) was refluxed for 20 hours with acetic anhydride (125 mL) and dry pyridine (125 mL). The anhydride and pyridine were then removed in vacuo and the residual liquid fractionated. The product (43 g), bp 115°C/15 mm., gave an amide, mp 157°C not depressed on admixture of the specimen with an authentic sample of phenylacetamide.
Benzyl cyanide (30 g) was refluxed for 9 hours with acetic anhydride (100 mL) and dry pyridine