A convenient method for the preparation of 1,2-dihydroxy-3,4-dimethoxybenzene (I) in quantity has now been found in the oxidation of the accessible gallacetophenone 3,4-dimethyl ether with H2O2 in alkaline solution, and it has been used in the synthesis of dill-apiole in the following manner. Treatment with allyl bromide in acetone in presence of K2CO3 yielded a monallyl ether, which underwent molecular rearrangement on heating with production of 1,2-dihydroxy-3,4-dimethoxyallylbenzene (II) (uncharacterised) as the chief product. Since migration of an allyl group under these conditions always yields an o- or a p-hydroxyallylbenzene, the production of (II) probably means that the monoallyl ether is chiefly the 2-O-allyl ether. Methylenation of (II) with CH2I2 and anhydrous K2CO3 in acetone yielded directly almost pure dill-apiole (III), characterised by the formation of monobromodill-apiole dibromide, which was directly compared (mixed mp.) with a specimen prepared from natural dill-apiole. The allyl groups in these compounds cannot occupy position 6, since the final product would then be an isomeride of dill-apiole and not dill-apiole itself, the constitution of which was definitely established by the researches of Ciamician and Silber1 and Thoms2. Dill-apiole has been isolated from dill oil1, from matico oil from the leaves of Piper augustifolium2, from sea-fennel oil3, and from Crithmum maritimum4.
Methylation of (II) with methyl sulphate and alkali readily gave 1,2,3,4-tetramethoxy-5-allylbenzene, which is apparently identical with a product isolated by Thoms5 from parsley oil and to which this consitution was assigned. Direct comparision of the synthetic and the natural product was not possible in this case.
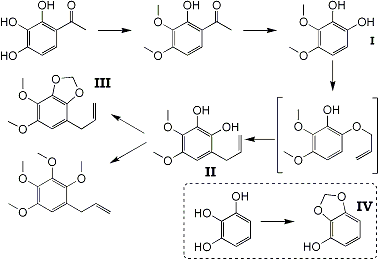
As possible intermediates in the synthesis of parsley apiole the following compounds have been prepared: pyrogallol methylene ether (2,3-methylenedioxyphenol) (IV), by the direct methylenation of pyrogallol with CH2Br2 and K2CO3 in acetone; gallacetophenone-4-methyl ether, by the nuclear acylation of pyrogallol 1-monomethyl ether6; 7-methoxy-8-acetyl-2-methylchromone - this compound could not be hydrolysed to the monomethyl ether of 2,4-diacetylresorcinol. Hydrolysis of 7-methoxy-8-acetyl-2-methylchromone7with 4% aq. NaOH at 100°C gives resacetophenone, the nuclear acetyl group in position 8 having been replaced by hydrogen; 2,4-diacetylresorcinol is unaffected under these conditions, although it has been recorded that reduction of this diacetyl resorcinol by Clemmensen's method yields 4-ethylresorcinol8.
Experimental
Gallacetophenone 3,4-dimethyl ether
To a boiling mixture of gallacetophenone (16.8g, 1 mol), benzene (400 mL) and anhydrous K2CO3 (55g) was added methyl sulphate (26.5g, 2.05 mol) in one portion, and the whole refluxed for 6 h with occasional shaking. After the addition of water (750 mL) and shaking, the benzene layer was separated and shaken several times with NaOH solution, and the alkaline extracts solidified. The precipitated gallacetophenone 3,4-dimethyl ether after crystallisation from MeOH had mp 75-77°C (yield 10g) (for previous methods of preparation from gallacetophenone, see Perkin9, Perkin and Wilson10, David and von Kostanecki11).
1,2-Dihydroxy-3,4-dimethoxybenzene (I)
Gallacetophenone 3,4-dimethyl ether (9.8 g) in 10% NaOH solution (40 mL) was oxidised by the addition of a 3% solution of hydrogen peroxide (75 mL) in an atmosphere of coal gas. Considerable rise of temp occurred and the mixture darkened. After about 30 min the solution was acidified, and extracted with ether, the extracts dried and distilled, and 1,2-dihydroxy-3,4-dimethoxybenzene obtained as a pale yellow oil (5 g), bp 160-170°C/20mmHg.
1,2-Dihydroxy-3,4-dimethoxy-5-allylbenzene (II)
To 1,2-dihydroxy-3,4-dimethoxybenzene (12 g) and allyl bromide (8.5 g) in acetone (25 mL) was added anhydrous K2CO3 (12 g), and the mixture refluxed for 18 hours with frequent shaking. After evaporation of the acetone, the mixture was acidified with dilute H 2SO4 and extracted with ether, the extracts were shaken with an excess of dil. aq. NaOH, and the phenolic products precipitated from the alkaline layer by acid and again extracted with ether. After removal of the solvent the monoallyl ether was heated in an oil bath to about 165°C; its temp then suddenly rose to 190°C, and, after being kept at 200°C for a few minutes, it was distilled at diminished pressure. The thick oily product (4 g), bp 160-173°C/14mmHg, which partly solidified was directly used in the following experiments.
Dill-Apiole (1,2-Methylenedioxy-3,4-Dimethoxy-5-Allylbenzene) (III)
Crude 1,2-dihydroxy-3,4-dimethoxy-5-allylbenzene (4 g), CH2I2 (5.2 g), acetone (25 mL) and anhydrous K2CO3 (3 g) were refluxed for 8 hours. After removal of the acetone and dilution of the residue with water, the methylenated product was extracted with ether, and the extracts shaken with dil. aq. NaOH and distilled twice under reduced pressure. The dill-apiole (1 g) distilled as a colourless, almost odourless oil at 172-173°C/16mmHg.
1,2,3,4-Tetramethoxy-5-allylbenzene
1,2-dihydroxy-3,4-dimethoxy-5-allylbenzene (3 gr) in MeOH (15 mL) was shaken with methyl sulphate (8 g) and a 10% solution of KOH (60 mL), added in portions, and finally heated on the steam-bath. After addition of water ether extracted the 1,2,3,4-tetramethoxy-5-allylbenzene as an oil, which after distillation at diminished pressure (bp 145°C/12mmHg) solidified when strongly cooled, and had an mp of 25°C.
Pyrogallol Methylene Ether (IV)
Pyrogallol (50 g), acetone (400 mL), Me2Br2 (110 gr) and anhydrous K2CO3 were refluxed for 30 hours, then diluted, acidified, and extracted with a large volume of ether. The ethereal extract was shaken several times with water, filtered, and shaken with aq. NaOH. The alkaline layer was acidified, extracted several times with warm light petroleum (bp 40-60°C), and yielded a semi-solid product which, after being presses on porous porcelain and recrystallised from light petroleum had mp 65°C.