Abstract
The combination of NaBH4 with catalytic quantities of NiCl2 smoothly reduces aliphatic nitro compounds to amines in methanol; Ni2B formed in situ is the active catalyst.
Recent advances in the alkylation of nitroaliphatic compounds have led to several new carbon-carbon bond forming strategies in organic synthesis1. For this chemistry to gain more widespread use, improved methods must be developed to modify or remove nitro groups. While nitroarenes can be reduced to anilines by many different techniques2, far fewer reagents are known which successfully reduce aliphatic nitro groups to their corresponding amines. Nitroaliphatic compounds have traditionally been reduced by high-pressure hydrogenation3, lithium aluminum hydride4 or aluminum amalgam5. Only in the last few years have alternative methods emerged6, including the use of transfer hydrogenation7, and titanium(II) reagents8,9.
Work in our laboratory on the mechanism of cobalt(II)-promoted NaBH4 and LiAlH4 reductions has shown that amorphous transition metal borides play an essential (and sometimes surprising) role as heterogeneous catalysts in the reduction of nitriles, alkenes and alkyl halides10. Although several diverse types of transition metal salt-hydride reagents are known to reduce nitroarenes11, we could find no published example of nitroalkane reductions by such systems. Nevertheless, it seemed worthwhile to ask whether one of the wore highly reactive borides might catalyze the hydride reduction of aliphatic nitro groups. Here we report that the combination of nickel boride (Ni2B) and NaBH4 in CH3OH smoothly reduces a variety of primary, secondary and tertiary nitroaliphatic compounds to amines within ca. 15 min at RT. The process is catalytic in boride, which is prepared in situ by the NaBH4 reduction of NiCl2 in CH3OH12.
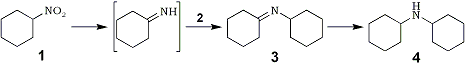
Preliminary experiments revealed that neither the boride alone nor boride under hydrogen could reduce nitrocyclohexane 1 to cyclohexylamine 212,13.
Table
NaBH4-Ni2B Reductions of Nitroaliphatic Compounds
Reactanta |
Productb |
(Yield)c |
1-Nitro-cyclohexene (1) | Cyclohexylamine (2) |
(68%)d |
1-Nitro-cyclohex-1-ene (5) |
(66%)d |
|
1-Nitrooctane (6) | n-Octylamine (7) |
(61%) |
5-Nitro-5-methyl- hexan-2-ol (8) | 5-Amino-5-methyl- hexan-2-ol (9) | (76%) |
4-Nitro-4-methyl- pentanoic acid (10) | Ammonium 4-Amino- 4-methyl-pentanoate (11) | (64%) |
Methyl 4-Nitro- 4-methyl-pentanoate (12) | 5,5-Dimethyl- pyrrolidinone (13) |
(50%)e |
- All reductions carried out according to the procedure below.
- Products identified by comparison with authentic samples.
Satisfactory IR, NMR and MS were obtained for all products. - Yields reported are for isolated, pure compounds.
- Isolated as HCl salt; lower yields due to its water solubility.
- Product stirred 2d at RT in CH3OH before workup.
However mixtures of Ni2B with NaBH4 in CH3OH rapidly reduced 1 to 2 in good yield, although the desired product was contaminated with variable amounts of dicyclohexylamine 4, depending on the quantity and preparation of catalyst. Compound 4 most likely arose from imine 3, generated in situ from 2 and transient amounts of cyclohexanone imine, a presumed reduction intermediate.
Dimer 3 seemed to be suppressed when freshly prepared, finely divided boride was used. Typically, a sonicated solution of NiCl2 in CH3OH was pre-reduced at rt using 1.0-1.5 equiv NaBH4. After sonicating for 30 min, the first-formed clumps of Ni2B were dispersed as a very finely divided precipitate. Best results (98:2 2:4) were obtained using 25 mol% of catalyst prepared in this fashion, along with 3-3.5 equiv of NaBH4 (see Table). Reductions of 1 using NaBH4-NiCl2 in 2-propanol/water or ethanol were considerably slower and gave several additional byproducts12. Methanolic NaBH4-cobalt boride (Co2B) failed to reduce nitroaliphatic compounds.
Experimental
The following preparation of 5-amino-5-methyl-2-hexanol is representative:
A 100 mL roundbottom flask containing NiCl2·6H2O (0.368g, 1.55 mmol) and CH3OH (30 mL) was sonicated to effect complete solution, then solid NaBH4 (0.176g, 4.65 mmol) was added portionwise (CAUTION: frothing). After 30 min, 5-nitro-5-methyl-2-hexanol (0.500g, 3.1 mmol) was added in CH3OH (2 mL) followed by more solid NaBH4 (0.410g, 10.9 mmol) over a 5 min period. Five minutes later, when thin layer chromatography indicated all the nitro compound had disappeared, the reaction mixture was filtered through Celite and the boride rinsed with CH3OH (10 mL). The combined filtrates were eluted onto a 20x100mm Dowex 50(H+) column previously equilibrated with CH3OH. After rinsing with more CH3OH (150mL), elution with 1.5 M NH3 in CH3OH (150 mL) afforded 0.309g (76%) of product aminoalcohol as a colorless, viscous oil.
In the case of less water-soluble amines, products could be isolated simply by evaporating the methanol filtrate and carrying out acid-base partition extraction.