Abstract
Reaction of aryl halides with sodium or potassium acetylacetonate in the presence of a copper catalyst affords
directly arylacetones resulted from deacetylation of initially formed
3-arylacetylacetones in good yields.
An investigation of a new efficient synthetic method of arylacetones gives us interesting useful intermediates for drug synthesis of central nervous agents. In this paper, we wish to report an efficient, direct synthesis of arylacetones from the reaction of aryl halides with acetylacetone.
Some preparations of arylacetones have been reported1-4. However, these reactions mostly need drastic conditions and a few or more steps, and generally afford the corresponding products only in moderate or poor yields. Hence, particularly, in manufacturing scale, they would be less useful. Thus, we were interested in the utility of acetylacetone as a reagent convertible easily to an acetonyl group and studied reactions with aryl halides.
The reaction of acetylacetone with aryl halides has been hitherto known only in cases of aryl halides substituted with a carboxy group in the ortho-position in the presence of Cu(II) derived from disproportionation of Cu(I)5-6. In such a case, it was presumed that a carboxylate anion would be concerned in reaction5.
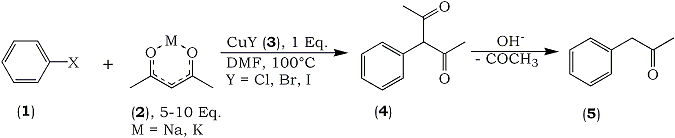
We investigated the direct arylation of acetylacetone with aryl halides without the aid of such a carboxyl group. Consequently, we could obtain 3-arylaetones from the reaction in the presence of Cu(I) halide. Since these 3-arylacetylacetones trend to deacetylate under alkaline condition, subsequent deacetylation in diluted alkaline solution in the same pot afforded the corresponding arylacetones in good yields. This procedure must be simple because two steps reaction can be performed in a one-pot.
Experimental
A typical procedure is as follows:
3-bromobenzophenone (5 mmol) was dissolved in 15 ml of dry dimethylformamide, then CuI (5 mmol) and potassium acetylacetonate (25 mmol)7 were added successively under a nitrogen current. The reaction mixture was heated for 6 hr at 100°C to afford a mixture of acetonylbenzophenonea (bp1.0 177-181°C), and 3-(3-benzoylphenyl)-acetylacetoneb, in 60 and 14% yields, respectively. The latter acetylacetone compound was quantitatively converted to 3-acetonylbenzophenone by treatment with dilute aqueous NaOH at room temp. After usual work-up, the products were purified by silica-gel column chromatography.
Spectral data:
a. vC = 0 1715, 1650; δ in CDCl3 2.16 (3H, s, CH3), 3.75 (2H, s, CH2), 7.30-7.85 (9H, m, ArH); M+ 238
b. vC = 0 1650, 1600; δ in CDCl3 1.86 (6H, s, CH3x2), 7.30- 7.80 (10 H, m, ArH and CH); M+ 280
Usually this procedure could be successively performed in the same reaction vessel without isolation of both compounds. The results obtained using various aryl halides under several different conditions are summarized in Table I.
| Entry | ArX (1) | M (2) | Y (3) | Time | 4a Yieldb | 5a Yieldb |
| 1 | 3-PhCOC6H4Br | K | I | 6.0 h | 14%c | 60%c |
| 2 | 3-PhCOC6H4Br | K | Br | 7.5 h | 80% |
|
| 3 | 3-PhCOC6H4Br | K | Cl | 11.0 h | 76% |
|
| 4 | 3-AcC6H4Br | Na | I | 15.0 h | 81% |
|
| 5 | 4-AcC6H4Br | Na | I | 15.0 h | 66% |
|
| 6 | 4-NO2C6H4Br | Na | I | 1.5 h | 43% |
|
| 7 | 3-F-4-PhC6H44Br | Na | I | 14.0 h | 67% |
|
| 8 | PhBr | Na | I | 30.0 h | 72% |
|
| 9 | 2-CH3C6H4Br | Na | I | 48.0 h | 32% |
|
| 10 | 4-CH3C6H4Br | Na | I | 23.0 h | 10%c | 65%c |
| 11 | 4-CH3C6H4Br | K | I | 9.0 h | 85% |
|
| 12 | 4-CH3C6H4I | K | I | 2.0 h | 82% |
|
| 13 | 4-CH3OC6H4Br | Na | I | 23.0 h | 26%c | 67%c |
| 14 | 4-AcNHC6H4Br | Na | I | 27.0 h | 82%d |
Notes:
a. Mp and bp of new product;
Entry 7 (5); mp 74.5-75.5°C, Entry 13 (4); bp7.0130-134°C
b. Isolated yield
c. Yield resulting from the initial reaction is shown
d. Used 2 equiv. of 3
As shown in Table I, it is indicated that the combination of cuprous iodide and potassium acetylacetonate gives the best result.
In this reaction, the differences of reaction conditions in changes of solvent, temperature, and catalyst would seriously affect the formation of product. The use of a protic solvent did not result in the formation of the desired product, but afforded the corresponding reduced product as a major product. Therefore, aprotic solvents having high solubility such as dimethylformamide should be desired in this reaction.
The reaction temperature over 120°C should be avoided because the decomposition of acetylacetonate might be caused.
Next, we found that the reaction proceeded not only in the presence of Cu+ halides as catalyst, but also in the reaction with Cu2+ acetylacetonate resulted from disproportionation. The catalytic abilities of Cu2+and Cu0 were also investigated for exploiting the reaction process.
Based on the information that the formation of Cu0 and Cu2+ acetylacetonate have been anticipated in our system, we investigated these catalytic abilities for the substitution reaction of acetylacetone with 3-bromobenzophenone. The reaction of 3-bromobenzophenone (1 mol. eq.) with or without potassium acetylacetonate (4 mol. eq.) was carried out in dimethylformamide at 100°C in the presence or absence of Cu0, Cu+ or Cu2+ as a catalyst. After the reaction, 3-(3-benzoylphenyl)acetylacetone formed was successively deacetylated by treatment with diluted NaOH aq. solution in the same vessel. These results are summarized in Table II.
| Run | Catalyst (Molar Ratio) | Time | Yielda of 5a |
| 1 | Cu2+(CHAc2)2- (1) | 24 h | no reactionb |
| 2 | --- | 24 h | tracec,d |
| 3 | Cu2+(CHAc2)2- (0.5) | 11 h | 61% |
| 4 | Cu2+(CHAc2)2- (0.5) + Cu0 (0.5) | 11 h | 61% |
| 5 | Cu2+(CHAc2)2- (0.5) + KI (1) | 7 h | 77% |
| 6 | CuI (1) | 20 h | 74%e |
| 7 | Cu0 (0.1) | 20 h | traced |
Notes:
a. Isolated yield
b. In the absence of 2
c. In the absence of a catalyst
d. Detected by TLC and GLC
e. Used 5 equiv. of 2
The substitution reaction could not completely take place with only Cu2+ acetylacetonate formed by disproportionation (Run 1). Furthermore, 3-bromobenzophenone was hardly substituted with only use of potassium acetylacetonate (Run 2). In addition, Cu0 exhibited littly catalytic activity (Run 7). Apparently, in the promotion of the present reaction, the presence of Cu+ and/or Cu2+ would be essential. The catalytic activity of Cu+ appears to be equal or greater than that of Cu2+, and the addition of potassium iodide may accelerate the rate due to the activation of Cu2+.