Summary
Friedel-Crafts α-(acyl)methylthiomethylations of aromatic compounds with α-ethoxycarbonyl, -acetyl, -benzoyl, and -cyano-α-(methylthio)methyl chlorides are described. The resulted products are easily converted to acylmethylated aromatics such as phenylacetate and phenylacetone by reductive desulfurization.
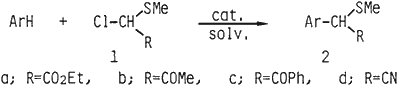
Direct acylmethylation of aromatic nucleus by Friedel-Crafts reaction with the acylmethyl halides such as ethyl chloroacetate1 and chloroacetone2 is generally difficult. This is presumably because the intermediary electrophilic complex of the Friedel-Crafts reaction is inactivated by the neighboring electronegative acyl-group. We found that the introduction of a methylthio group to the acylmethyl halide greatly enhances the reactivity of the electrophilic complex. Thus, the Friedel-Crafts reaction with α-ethoxycarbonyl-α-(methylthio)methyl chloride (1a) and the related α-acetyl, α-benzoyl, and α-cyano-α-(methylthio)-methyl chlorides (1b-d) afforded a general method for introduction of an α-(acyl)-methylthiomethyl group into the aromatic nucleus. The present method provides also a useful synthetic way to the acylmethylated aromatic compound such as arylacetate or arylacetone because of the easily removable character of methylthio group of the resulted products (2a-d).
Table. Friedel-Crafts Reactions of Aromatic Compounds with 1a-d
Halide |
Aromatic (eq)a | Cat. |
Solv.b | temp |
Time (min) |
Yield of 2 |
1a |
benzene (5) | SnCl4 | — | r.t. | 20 |
91% |
1a |
benzene (1) | SnCl4 | CH2Cl2 | r.t. | 40 |
87% |
1a |
isobutylbenzene (1) | SnCl4 | CH2Cl2 | r.t. | 40 | quant. (p-) |
1a |
chlorobenzene (1) | SnCl4 | CH2Cl2 | reflux | 90 |
90% (o:p=1:4) |
1a |
naphthalene (1) | SnCl4 | CH2Cl2 | r.t. | 40 | quant. (α-) |
1a |
thiophene (5) | ZnCl2 | CH2Cl2 | 0°C | 40 |
53% (α-) |
1a |
ethyl benzoate (1) | SnCl4 | CH2Cl2 | reflux | 90 |
0% |
1b |
benzene (5) | SnCl4 | — | r.t. | 40 |
88% |
1c |
benzene (5) | SnCl4 | — | r.t. | 40 |
75% |
1d |
benzene (5) | SnCl4 | — | r.t. | 40 |
82% |
a) arom.compd./1 b) molecular proportions of solvent and 1a were 5:1.
The α-acyl-α-(methylthio)- methyl chlorides (1a-d) were prepared from the corresponding α-(acyl)- dimethyl sulfides by treating with N-chloro- succinimide.3 The Friedel- Crafts reaction of the aromatic compounds with 1 was successfully carried out in methylene chloride or in excess aromatic compounds under the mild conditions in the presence of one equivalent4 of Friedel-Crafts catalyst (AlCl3, SnCl4, TiCl4 etc). The results are summarized in Table.
The Friedel-Crafts products 2a-d thus obtained were readily desulfurized by treatment with Raney nickel or zinc dust-acetic acid to produce aryl-acetate, -acetone, -acetophenone, and -acetonitrile, respectively, in high yield.
Experimental
The following procedure for preparation of phenylacetone is representative.
Stannic chloride (1.03 g, 3.96 mmol) was added to a stirred solution of 1b (549 mg, 3.96 mmol) in benzene (2 ml) at room temperature. The reaction mixture was stirred at the same temperature for 20 min. The mixture was poured into water and extracted with benzene, and dried. The solvent was evaporated off, and the residue was chromatographed on silica gel, using benzene as an eluent, to give α-(methylthio)phenylacetone (2b, Ar=Ph) in yield of 88% (630 mg).
Zinc dust (2.5 g, 38 mmol) was added to a stirred solution of the above compound (521 mg, 2.89 mmol) in acetic acid (3 ml) at room temperature. The reaction mixture was stirred at 100°C for 1 hr. Water (20 ml) and methylene chloride (30 ml) was added to the mixture, and the inorganic materials were filtered off, and then the aqueous layer was further extracted with methylene chloride. The organic layers were combined and dried. Evaporation of the solvent and distillation of the residue gave phenylacetone in yield of 94%.