Although many of the known hallucinogens possess centres of optical asymmetry, in most cases they have been evaluated only in their racemic state. An exception is the highly potent psychotomimetic (+)-LSD, which has two asymmetric centres. The absolute configuration about each has been established3 as 5-R; 8-R (I). Two diastereoisomeric diethyllysergamides exist with the opposite configuration at C5; vis., (-)-LSD (5-S; 8-S) and (-)-isoLSD (5-S; 8-R). Both are reported to be without hallucinogenic effect in man5.
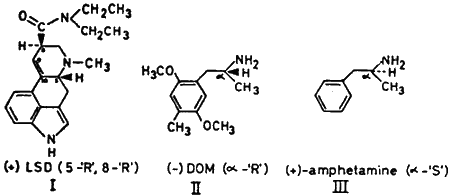
Many optically active stimulants and anorexogenic agents are of established absolute configuration, and when compared with the phenyl-C10-C5-N6 chain of (+)-LSD are of the opposite, or S configuration. Examples are (+)-amphetamine III2 and (-)-ephedrine4.
Both (+)-benzphetamine and (±)-phendimetrazine may be derived from the above compounds and so maintain this configuration.
A number of compounds chemically related to the phenethylamines resemble (+)-LSD in their pharmacology6. These substances are α-methylphenethylamines and have an asymmetric carbon centre analogous to both amphetamine and to (+)-LSD, but which have heretofore been clinically evaluated only as racemates.
I now report the resolution and evaluation in man of the highly active hallucinogenic compound 2,5-dimethoxy-4,α-dimethylphenethylamine, DOM or STP (II). Employing circular dichroism, the absolute configurations of the optical enantiomers have been determined as being (+)-S and (-)-R in comparison to the corresponding unsubstituted amphetamines (Castagnoli, Neal, pers. comm.). In man the racemic compound is effective at a threshold level of about 1 mg total dose (16 µg·kg-1, orally)6. At levels of above 50 µg·kg-1 the central sensory effects become marked7 and it is only at very high levels (above 200 µg·kg-1)1 that indications of amphetamine-like stimulation appear.
The laevorotatory R form of II is the hallucinogenic isomer. An acute dose of 8 µg·kg-1 of (-)-R-DOM was equivalent to twice its weight of racemate both in the nature and duration of the subjective effects experienced. The (+)-S-isomer was without central effects at many times this dose. Even at 30 µg·kg-1 no signs of sympathomimetic stimulation were noted (mydriasis, hypertension, tachycardia) and at no level could the central hallucinogenic syndrome of either the racemate or the laevorotatory isomer be evoked.
It is of interest that the R isomer of DOM and the isomer of LSD with the R configuration at C5 are the effective hallucinogens. Taken with the inactivity of the S-counterparts, the observations provide circumstantial evidence of a selective and asymmetric site associated with the mechanisms of the action of these drugs.