08-22-00 11:54
No 43910
Hi
Thought I'd posted this question last night; but since was suffering from serious sleep deprivation @ the time, who knows what happened. But if it appears twice, apologies in advance.
In TCBOE the author (briefly) makes reference to a Japanese patent for producing p2p from alpha-methyl styrene, bromine, & sulfuric acid. Now, if alpha-methyl styrene were, as the author states, 1-phenyl-1-propene, this would be just another in the 'allylbenzene' series (1-phenyl-1-propene being to p2p as is isosafrole to safrole); but I've never heard anyone make mention of the fact that the author is in error. Alpha methyl styrene is 2-phenyl-1-propene; and that little alteration apparantly makes all the difference in the world, because my local chem supply house was able to price it for me @ $35/kg, & when I asked whether, when sending one of my employees down to pick up such an order (if I decided to place it), the employee would need to provide identification etc. in the case of that particular chemical, I was told that it was not Listed or Suspicious, & that they would gladly sell it to me anonymously.
Now, am I missing some salient point, or does this sound really cheap & easy? I mean, sulfuric acid & bromine are not exactly the rarest chems in the world... but does anyone have more information on this process? I've searched for an English-language equiv. to the Japanese patent, but w/out success (even coughed up some dough for STN; at the use of which, however, I am admittedly no expert). Any help would be appreciated (or directions to somewhere where this entire subject has been formerly hashed out at length).
Thanks.
jen
"I harbor for good or bad,
I permit to speak at every hazard..."
-whitman
(Hive Bee)
08-22-00 18:24
No 44009
CH, can you post the Japanese patent number? I can get a copy of it. I know some people that speak Japanese and can look at it. I might also be able to find a Japanese chemist if I need one.
(Stranger)
08-23-00 04:16
No 44197
That sounds great... the ref is: Inoi, Japan, 69 09,892 (1969); Brief abstract in CA v71, 61016x (1969).
Hope ya find it!
jen
jen
"I harbor for good or bad,
I permit to speak at every hazard..."
-whitman
(Hive Bee)
08-25-00 20:55
No 45378
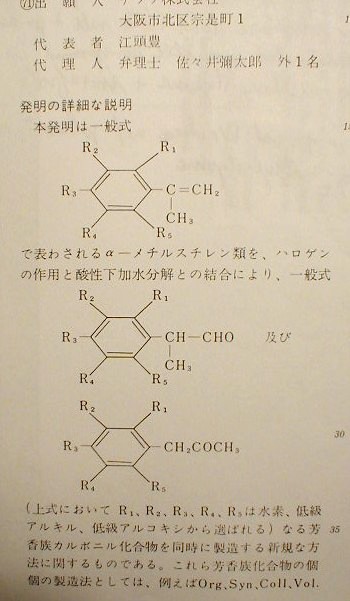
CH, I copied CA61016x and the Japanese patent. It looks like they're starting with alpha-methylstyrene and making alpha-phenylpropionaldehyde and methyl benzyl ketone from it. The Japanese patent has 5 examples. It looks like example 1 is described in CA61016x. I had three native Japanese speakers look at it and verbally translate the important parts for me. They don't know chemistry but we could still figure it out to some extent. The alpha-methylstyrene in CA61016x isn't a misprint. The chemical structure (with R1-R5 ring substituents) is shown in columns 1, 2 and 6, along with the phenylpropionaldehyde and methyl benzyl ketone products. Here's an image of the bottom part of column 1 of the Japanese patent showing the chemical structures. I've had some trouble with images from geocities but they seem to be working now. I'll try to fix it if the images stop working again.
(Stranger)
09-05-00 14:05
No 49071
Hey, thanks a lot! I'm sorry it took so long to reply to your reply, but I've been out of town (an affair of the heart ;-)), & I just got back in late last night. I'm going to send you a private message, tho... looking fwd to what you've been able to dig up!
jen
"I harbor for good or bad,
I permit to speak at every hazard..."
-whitman
(Stranger)
09-14-00 20:12
No 52305
The phenylpropionaldehye could easily be obtained from the a-methylstyrene by wacker oxidation. Conversion to the final P2P is detailed in TSII. I could write up the segment for you if you want.
A better but more hazardous way to get to P2P from a-methylstyrene is to using thallium nitrate (II or III; can't remember) in methanol to catalyze the isomerization. The reaction is VERY quick (under 10 minutes) and can easily be observed through the precipitation of some thallium salt, the name of which I can't remember at this time. The yields are somewhere around 90% and judging by what I've read about the proceedure, as long as you have all three reagents: a-methyl-styrene, methanol, and the thallium nitrate salt, you're good to go.
The only catch is the hazards involved in working with thallium nitrate (and of course, it's expense). But everything is legal to obtain, and since the thallium is not aromatic, I would think that as long as you wore gloves and were careful not to breath the stuff, you'd be okay.
A better, not quite as easy but easy enough, non-suspicious and much more safe way to get to P2P is through the following reaction:
Priopiophenone is smoothly reduced to phenyl-1-propanol via sodium borohydride in absolute ethanol solvent (15 minute addition time and 15 minute reaction time). After standard work-up and isolation, dehydration of the alcohol using potassium bisulfate followed by direct vaccum distillation yields the propenylbenzene (all in one straight-forward step without any need to purify any of the intermediates). Oxidation of the propenylbenzene into the diol ala the performic reaction, followed by dehydration, yields the desired P2P.
For more info, look under Rhodium's page.
I have references for all of the above proceedures. Just ask.
--PK
(Chief Bee)
09-18-00 10:51
No 53169
Hi kitty!
I would very much like to have a writeup for this reaction sequence on my page.
http://rhodium.lycaeum.org
(Hive Bee)
09-18-00 22:38
No 53326
such a pain in the ass just to get meth if u ask me. But I do like the creativity i see in the forum. Do you know if the thallium rxn works with the methylenedioxy counterparts?
alphabeta121
(Stranger)
10-12-00 13:25
No 59527
Hello,
I know I'm a newbee and I'm really must be asking for trouble by posting in the serious forum but I'd just like to know if I have the right understanding of this theory.
So the vicinal dihalide is obtained essentially through allylic bromination. Ice cold bromine dissolved in DCM would be added dropwise to the ams at a rate so no excess of bromine accumulates.
Once the dihalide compound is formed, it would then crystalize out of the DCM. The DCM would be filtered off and the crystals dried under vacuum.
The dihalide would then be refluxed in 15% solution of conc H2SO4 and methanol.
Somehow the dihalide forms a diol.(I not quite getting this part so if some could explain the mechanism I'd appreciate it) Anyway the diol undergoes a pinacol rearrangement in the acid media and forms the ketone and the aldehyde?
(Stranger)
10-12-00 19:18
No 59644
Which reaction sequence in particular, although kitty has to warn you that SWIM has actually never tried any of the proceedures of which you want a write-up. Just have the references.
--PK
(Stranger)
10-12-00 19:20
No 59646
According to a U.S. Patent that kitty has, yes, the thallium proceedure does work on methylendioxy compounds and the like; however, one would need to have 3,4-methylenedioxy-a-methylstyrene to get it to work, which to kitty's knowledge, isn't commercially available.
--PK