(Hive Addict)
06-06-03 00:23
No 438080
(Rated as: excellent)
A re-examination of the methylenation reaction
Maria Grazia Cabiddu, Enzo Cadoni, Stefania De Montis, Claudia Fattuoni,
Stefana Melis* and Michele Usai
Dipartimento di Scienze Chimiche, Cittadella Universitaria di Monserrato, SS 554 Bivio per Sestu, I-09042 Monserrato (CA), Italy
Received 3 January 2003; revised 18 March 2003; accepted 10 April 2003
Abstract - A re-examination of the methylenation reaction of 1-hydroxy-2-mercapto-, 1,2-dihydroxy- and 1,2-dimercapto substituted benzenes by bromochloromethane with cesium carbonate shows that these substrates give mixtures of five- and ten-membered benzocondensed heterocyclic compounds and in some cases even dibenzodioxines. 2003 Elsevier Science Ltd. All rights reserved.
In previous papers we reported a systematic study on bimetallation of aromatics promoted by the thioethereal group. Recently, this research has been extended to 1,3-benzodioxoles and 1,3-benzoxathioles1,2 because these systems are contained in natural compounds, in bioactive molecules and in materials showing non-linear optical properties.3-8
It is known that these compounds can be prepared condensing 1,2-benzodioles or 2-hydroxybenzenethioles with dichloromethane or dibromomethane in the presence of alkaline hydroxides or fluorides or potassium carbonate or sodium hydride in various solvents (DMSO, DMF, HMPA) with or without catalytic amounts of bronze, nickel oxide or cupric oxide.9-15 Good results can be obtained using phase transfer catalysts. 16,17 More recently, the methylenation of a variety of catechols has been reported, employing cesium carbonate and bromochloromethane in N,N-dimethylformamide or acetonitrile. 18
In this work we intend to verify if this method can be applied to the methylenation of 2-hydroxybenzenethiol (1a).
All reactions were performed in anhydrous N,N-dimethylformamide reacting 1a, cesium carbonate and bromochloromethane. Reaction yields were determined by HPLC analysis of the reaction mixture obtained after extraction with solvents. The products were isolated as pure by flashchromatography (Scheme 1).
Surprisingly, the expected 1,3-benzoxathiole (2a), liquid at room temperature, 17 was obtained in a 32% yield, while the main product (65% yield) was a solid with mp 148–150°C: its elemental analysis was equal to 2a, but its mass spectrum showed a molecular ion M+ of 276, corresponding to the dimer 3a. Furthermore, the 1NMR spectrum of 2a showed the methylene singlet at d 5.50, 17 while the solid product showed two singlets of equal intensity at d 4.12 and 5.89 attributable to the two methylenes in 3a.
For a better understanding of this methylenation reaction, we decided to re-examine the reactions with catecholic derivatives. Using 1,2-benzodiol and its derivatives the reaction gave a mixture of benzodioxole and tetraoxecine derivatives. In detail, methyl derivatives 1c and 1d gave a significant amount of tetraoxecines ranging between 26 and 30%: in fact 1c gave a 1.1:1 mixture of two isomeric tetraoxecines 3c and 3’c; while 1d gave an almost equimolar mixture of 3d and 3’d. Employing the methoxy derivative 1g, we obtained a mixture of the benzodioxole 2g (68%) and the isomeric tetraoxecine 3g and 3’g (17%) beside two isomeric dibenzodioxines 4 (or 4’) and 5 (or 5’), in almost equal amounts (Fig. 1).
Then we decided to apply this reaction protocol to 1,2-dimercaptobenzene (1h): 1,3-benzodithiole (2h) (98%) was obtained almost exclusively beside traces amount of unknown products.
The same reactions performed in acetonitrile and at different concentrations gave analogous results. At the end we can deduce that when the benzene ring bears two equally nucleophilic groups (benzodioles and dimercaptobenzenes) the main product is the one with a five-membered ring; when the groups have a different nucleophilic power the main product is the ten-membered ring.
The structure determination of all products was performed by comparison with commercial authentic samples or by spectroscopic techniques. For example all 3 compounds show a mass spectrum with a similar fragmentation pattern, with a low abundance molecular ion and a base peak represented by a ion I or II, both of which have greater aromatic character than the molecular ion (Fig. 2).
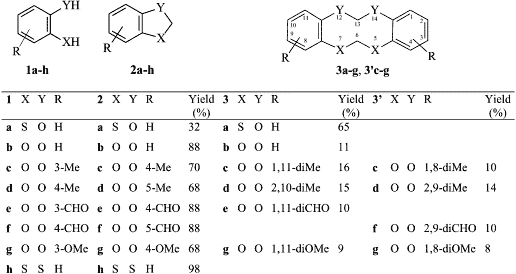
Scheme 1.
Two isomers 3c and 30c were identified by 1H NMR analysis of their mixture. The HPLC analysis revealed two peaks with very close retention times (4.38 and 4.59 min, respectively) so that their separation appeared to be very difficult. The 1H NMRspectrum of their mixture showed two singlets at d2.26 and 2.28 in the ratio of 1:1.1, attributable to the methyl groups of the two isomers; two singlets at d5.57 and 5.62 were due to the non-equivalents methylenes of the more abundant isomer 3c and another singlet at d 5.56 was attributed to the two methylenes of the isomer 3’c.
For 3d and 3’d the HPLC analysis of their mixture revealed only one peak. The two isomers were identified by the 1H NMR spectrum of their mixture: two singlets at d 5.61 and 5.65 were attributed to the two non-equivalents methylenes of the more abundant isomer 3d and a singlet at d 5.63 was attributed to the two methylenes of 3’d. The relative intensity of these signals revealed a ratio between the two isomers 3d/3’d of 1.1:1. The methyl groups of the two isomers gave only one signal at d 2.33.
Following the reaction of 1g chromatography gave 2g and an inseparable mixture of 3g and 3’g. Also isolated was a mixture of isomeric dibenzodioxines. The compound 3g was identified by two singlets, due to the methylenes, at d 5.57 and 5.73 in the 1H NMR spectrum, while another singlet at 5.85 was attributed to the equivalent methylenes of 3’g. On the contrary, we were unable to separate the two dibenzodioxines 4 and 5 because their HPLC retention times were too close (5.05 and 5.3 min, respectively): then we performed several successive flash-chromatographies to enrich the mixture in one isomer. We reached a mixture composition of 5:1 and its elemental analysis and mass spectrum were identical to the ones of the 1:1 mixture. The mass spectrum showed the molecular ion as base peak and a very poor fragmentation, typical of aromatic compounds. The enrichment made easier the assignment of some signals in the 1H and 13C NMR spectra, but it was not possible to attribute them to one of the four possible structures. The formation of the benzodioxines was completely unexpected: in fact these compounds are usually obtained through nucleophilic substitution by phenoxide anion on aryl halides or by self-condensation of 2-halogenophenols.19,20 We may tentatively justify the formation of these benzocondensed compounds by a radical process involving a semiquinone radical, which can easily be formed in alkaline solution by oxidation. This anion radical then attacks the already formed benzodioxole leading to the condensed 4 (or 4’) and 5 (or 5’).
The molecular ion of 3’f gave a particularly low abundance peak because the two CHO in para to the OCH2O led to a predominance of the symmetric fragmentation showed in the following structure (Fig. 3).
Figure 2
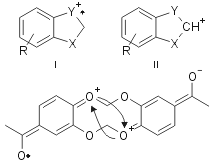
Figure 3
The faster you run, the quicker you die.
(Hive Addict)
06-06-03 00:26
No 438081
Experimental
1.1. General
Analyses by HPLC were carried out with a Waters 600 HPLC equipped with a UV Gilson 116 detector and a Spherisorb NH2 (in the case of aldehydes a Spherisorb silica) 5m column (25 cm x 4.6 mm) using 98:2 hexane/2-propanol as eluent. IR spectra were recorded on a Perkin–Elmer 1310 grating spectrophotometer. 1H and 13C NMR spectra were recorded on a Varian VXR-300 spectrometer. Mass spectra were obtained at 70 eV with a Hewlett–Packard 5989A mass spectrometer, using the direct inlet system. Microanalyses were carried out on a Carlo Erba 1106 element analyser.
1.2. Materials
Reagent-grade commercially available reagents and solvents were used. 2-Hydroxybenzenethiol (1a), 1,2-benzodiol derivatives (1b–f) and 1,2-dimercaptobenzene (1g) were purchased (Aldrich).
1.3. General method of methylenation of compounds 1a–h
To a suspension of the starting compound 1a–h (50 mmol), cesium carbonate (24.4 g, 75 mmol) and anhydrous N,N-dimethylformamide (120 mL), was added dropwise bromochloromethane (9.6 g, 75 mmol) and the resulting mixture was vigorously stirred under a nitrogen atmosphere and then heated at 107–110°C for 2 h. The mixture was poured into water, the organic layer separated and the aqueous layer extracted with ether and then with dichloromethane, because ether is able to extract only the monomeric product while the dimeric ones are extracted by dichloromethane. The organic phases were dried (Na2SO4). The solvent was evaporated in vacuo and the residue was analysed by HPLC.
1.3.1. Methylenation of 1a. The HPLC analysis showed the presence of two products. The extract was flash-chromatographed using light petroleum as eluent. The first fraction was identified as 2a by comparison of its NMR and mass spectra with those of an authentic sample; yield 32%; pale yellow oil, bp 101–102°C/10 mm Hg (lit.17 bp 93–95°C/5 mm Hg). pale yellow oil, bp 65–66°C/10 mm Hg (lit.21 bp 60°C/9 mm Hg).
The second fraction was identified as 3b by comparison with an authentic sample; yield 11%; crystallised from acetic acid as white crystals, mp 262°C (lit.21 mp 261–262°C). [Found: C, 66.78; H, 4.91. C14H12O4 requires C, 66.85; H, 4.95%]; nmax (Nujol) 1590, 1500, 1250, 1195, 1105 cm-1; dH (300 MHz, CDCl3) 5.61 (4H, s, OCH2O), 7.11 (8H, s, ArH); dc (75.4 MHz, CDCl3) 98.5, 121.4, 125.7, 150.0. m/z (EI) 244 (6, M+), 135 (3), 122 (100), 121 (68), 80 (11), 77 (6).
1.3.3. Methylenation of 1c. The HPLC analysis showed three peaks: two peaks had very close retention times (4.38 and 4.59 min, respectively) so that their separation appeared to be very difficult.
The extract was flash-chromatographed using light petroleum as eluent. The first fraction was identified as 2c by comparison of its NMR and mass spectra with those of an authentic sample; yield 70%; pale yellow oil, bp 60°C/3 mm Hg (lit.22 bp 45°C/1.2 mm Hg). The 1H NMR analysis of the second fraction showed the presence of two isomers 3c and 3’c in a 1.1:1 ratio; total yield 26%; crystallised from acetic acid as white crystals, mp 190–192°C. [Found C, 70.48; H, 5.87. C16H16O4 requires C, 70.57; H, 5.92%]; nmax of the mixture (Nujol) 1570, 1515, 1250, 1200, 1115 cm-1.
Compound 3c. dH (300 MHz, CDCl3) 2.28 (6H, s, CH3), 5.57 (2H, s, OCH2O), 5.62 (2H, s, OCH2O), 7.08 (6H, m, ArH); dc (75.4 MHz, CDCl3) 20.5, 98.1, 98.3, 120.2, 122.4, 126.0, 135.2, 147.0, 150.3.
Compound 3’c. dH (300 MHz, CDCl3) 2.26 (6H, s, CH3), 5.56 (4H, s, OCH2O), 7.08 (6H, m, ArH); dc (75.4 MHz, CDCl3) 20.5, 98.2, 121.5, 122.6, 125.3, 136.4, 146.6, 150.7; m/z (EI) of the mixture: 272 (7, M+), 149 (4), 137 (9.5), 136 (100), 135 (53), 105 (3), 91 (6), 79 (5), 78 (8), 77 (10).
1.3.4. Methylenation of 1d. The HPLC analysis showed two peaks. The extract was flash-chromatographed using light petroleum as eluent. The first fraction was identified as 2d by comparison of its NMR and mass spectra with those of an authentic sample; yield 68%; pale yellow oil, bp 115–117°C/3 mm Hg (lit.21 bp 196–197°C/753.5 mm Hg).
The 1H NMR analysis of the second fraction (corresponding to the second HPLC peak) showed the presence of two isomers 3d and 3’d in a 1.1:1 ratio; total yield 29%; crystallised from acetic acid as white crystals, mp 219–220°C. [Found C, 70.50; H, 5.98 C16H16O4 requires C, 70.57; H, 5.92%]; nmax of the mixture (Nujol) 1580, 1505, 1255, 1205, 1120 cm-1.
Compound 3d. dH (300 MHz, CDCl3) 2.33 (6H, s, CH3), 5.61 (2H, s, OCH2O), 5.65 (2H, s, OCH2O), 6.97 (2H, d, J=8.1 Hz, ArH), 6.99 (2H, s, ArH), 7.06 (2H, d, J=8.1 Hz, ArH); dc (75.4 MHz, CDCl3) 20.9, 98.4, 98.6, 120.9, 121.8, 126.2, 135.6, 147.6, 149.5.
Compound 3’d. dH (300 MHz, CDCl3) 2.33 (6H, s, CH3), 5.63 (4H, s, OCH2O), 6.97 (2H, d, J=8.1 Hz, ArH), 6.99 (2H, s, ArH), 7.06 (2H, d, J=8.1 Hz, ArH); dc (75.4 MHz, CDCl3) 20.9, 98.5, 121.1, 121.6, 126.1, 135.5, 147.4, 149.6; m/z (EI) of the mixture 272 (8, M+), 149 (2), 137 (9), 136 (100), 135 (25), 106 (3), 91 (2), 78 (5), 77 (3).
1.3.5. Methylenation of 1e. The HPLC analysis showed the presence of two products. The extract was flash-chromatographed using 10:1 hexane/ethyl acetate as eluent. The first fraction was identified as 2e by comparison of its NMR and mass spectra with those of an authentic sample; yield 88%; crystallised from ethanol as white crystals, mp 32–34°C (lit.23 mp 33°C).
The second fraction was identified as 3e. Yield 10%, crystallised from acetic acid as a white solid, mp 200–202°C. [Found C, 63.91; H, 3.97. C16H12O6 requires C, 63.99; H, 4.03%]; nmax (Nujol) 2710, 1695, 1600, 1255, 1105 cm-1; dH (300 MHz, CDCl3) 5.69 (2H, s, OCH2O), 5.79 (2H, s, OCH2O), 7.31 (2H, t, J=7.5 Hz, ArH), 7.42 (2H, d, J=7.5 Hz, ArH), 7.65 (2H, d, J=7.5 Hz, ArH), 10.18 (2H, s, CHO); dc (75.4 MHz, CDCl3) 98.4, 99.1, 121.2, 122.7, 124.2, 132.4, 136.8, 148.9, 151.2, 190.1; m/z (EI) 300 (14, M+), 270 (5.5), 164 (15), 163 (15), 151 (17), 150 (91), 149 (100), 134 (23), 122 (14), 121 (24), 107 (15), 105 (13), 92 (21), 91 (10), 77 (18).
1.3.6. Methylenation of 1f. The HPLC analysis showed the presence of two products. The extract was flash-chromatographed using 10:1 hexane/ethyl acetate as eluent. The first fraction was identified as 2f by comparison of its NMR and mass spectra with those of an authentic sample; yield 88%; crystallised from ethanol as white crystals, mp 36–37°C (lit.24 mp 37°C).
The second fraction was identified as 3’f. Yield 10%; crystallised from acetic acid as white crystals, mp 260–262°C. [Found C, 63.88; H, 3.98. C126H12O6 (sic) requires C, 63.99; H, 4.03%]; nmax (Nujol) 2715, 1695, 1600, 1260, 1100 cm-1; dH (300 MHz, CDCl3) 5.70 (4H, s, OCH2O), 7.37 (2H, d, J=6.9 Hz, ArH), 7.65 (4H, m, ArH), 9.90 (2H, s, CHO); dc (75.4 MHz, CDCl3) 98.6, 122.0, 128.8, 130.9, 134.4, 154.7, 167.7, 190.2; m/z (EI) 300 (3, M+), 151 (10), 150 (100), 149 (79), 135 (2), 121 (14), 103 (3), 91 (5), 79 (10), 77 (6).
1.3.7. Methylenation of 1g. The HPLC analysis showed four peaks. The mixture was flash-chromatographed using 9:1 ether/light petroleum as eluent. The first fraction was identified as 2g by comparison of its NMR and mass spectra with those of an authentic sample; yield 68%; crystallised from ethanol as white crystals, mp 41–42°C (lit.15 mp 39–41°C).
The 1H NMR analysis of the second fraction (corresponding to the second HPLC peak) shows the presence of two isomers 3g and 3’g in a 1.1:1 ratio; yield 17%; crystallised from methanol as white crystals, mp 205–206°C. [Found C, 63.07; H, 5.35. C16H16O6 requires C, 63.15; H, 5.30%]; nmax of the mixture (Nujol) 1600, 1500, 1250, 1150 cm-1.
Compound 3g. dH (300 MHz, CDCl3) 3.77 (6H, s, OCH3), 5.57 (2H, s, OCH2O), 5.73 (2H, s, OCH2O), 6.79 (4H, m, ArH), 7.11 (2H, m, ArH); dc (75.4 MHz, CDCl3) 55.8, 94.5, 98.2, 108.4, 113.3, 124.8, 128.3, 151.1, 152.8.
Compound 3’g. dH (300 MHz, CDCl3) 3.80 (6H, s, OCH3), 5.85 (4H, s, OCH2O), 6.42 (4H, d, J=8.0 Hz, ArH), 6.68 (2H, t, J=7.8 Hz, ArH); dc (75.4 MHz, CDCl3) 56.4, 101.0, 102.3, 107.4, 121.9, 135.1, 143.9, 148.6; m/z (EI) of the mixture 304 (18, M+), 165 (6), 153 (10), 152 (100), 151 (34), 137 (8), 107 (13), 95 (10), 79 (6).
The third fraction was identified as a 1:1 mixture of two products 4 (or 4’) and 5 (or 5’). Yield 10%. This mixture was isolated by chromatography as a white solid with mp 219–220°C. [Found C, 62.41; H, 4.25. C15H12O6 requires: C, 62.50; H, 4.20%]; m/z (EI) 288 (100,M+), 273 (9), 245 (12), 243 (9), 230 (8), 217 (17), 215 (10), 187 (5), 165 (6), 144 (8), 116 (5), 77 (12).
After repeated column chromatographies on silica using light petroleum as eluent the mixture was enriched in one of the two components reaching a ratio of 5:1. The mp of this mixture was 232–234°C. The elemental analysis and mass spectrum of the 5:1 mixture were identical to those of the 1:1 mixture. One product: dH (300 MHz, CDCl3) 3.86 (3H, s, OCH3), 4.08 (3H, s, OCH3), 5.85 (2H, s, CH2), 6.15 (1H, s, ArH), 6.43 (1H, dd, J=8.0, 1.8 Hz, ArH), 6.54 (1H, dd, J=8.0, 1.8 Hz, ArH), 6.82 (1H, t, J=8.0 Hz, ArH); dc (75.4 MHz, CDCl3) 56.11, 60.50, 91.82, 101.04, 107.22, 109.87, 122.82, 128.99, 131.47, 132.78, 133.12, 137.08, 142.47, 143.41, 148.23. Other product: dH (300 MHz, CDCl3) 3.87 (3H, s, OCH3), 4.05 (3H, s, OCH3), 5.86 (2H, s, CH2), 6.31 (1H, s, ArH), 6.44 (1H, dd, J=8.2, 1.7 Hz, ArH), 6.54 (1H, dd, J=8.2, 1.7 Hz, ArH), 6.83 (1H, t, J=8.2 Hz, ArH); dc (75.4 MHz, CDCl3) 56.12, 60.51, 92.45, 101.06, 107.22, 109.25, 122.82, 128.98, 131.45, 132.79, 133.11, 137.08, 142.45, 143.41, 147.87.
1.3.8. Methylenation of 1h. The HPLC analysis showed the presence of 2h. The residue was flash-chromatographed using light petroleum as eluent. The first fraction was identified as 2h by comparison of its NMR and mass spectra with those of an authentic sample; yield 98%; pale yellow oil, bp 104–106°C/2 mm Hg (lit.25 bp 95–96°C/1 mm Hg).
We were not able to identify the trace amount products.
Financial support from the Ministero dell’Universita` e della Ricerca Scientifica e Tecnologica, Rome, (National Project ‘Stereoselezione in Sintesi Organica, Metodologie ad Applicazioni’), C.N.R. (Italy), University of Cagliari and Regione Autonoma della Sardegna, is gratefully acknowledged.
References
1. Cabiddu, S.; Cadoni, E.; Melis, S.; Gelli, G.; Cabiddu, M. G.; Fattuoni, C.; De Montis, S.; Ianelli, S. Tetrahedron 2001, 57, 10365–10375.
2. Cabiddu, S.; Cadoni, E.; Ianni, A.; Gelli, G.; Melis, S.; Bernard, A. M.; Cabiddu, M. G.; De Montis, S.; Fattuoni, C. Eur. J. Org. Chem. 2002, 3393–3401.
3. Snieckus, V. Chem. Rev. 1990, 90, 879–933, and references cited therein.
4. Lajide, L.; Escoubas, P.; Mizutani, J. J. Agric. Food Chem. 1993, 41, 2426–2430.
5. Friedrich, M.; Meichle, W.; Bernhard, H.; Rihs, G.; Otto, H. H. Arch. Pharm. Pharm. Med. Chem. 1996, 329, 361–370.
6. Venkata Rao, D.; Omprakash, G.; Subrahmanyam, C. Indian J. Chem. 1996, 35B, 1349–1351.
7. Majerus, S. L.; Alibhai, N.; Tripathy, S.; Durst, T. Can. J. Chem. 2000, 78, 1345–1355.
8. D’Arcangelis, S. T.; Cowan, D. O. Tetrahedron Lett. 1996, 37, 2931–2934.
9. Campbell, K. N.; Hopper, P. F.; Campbell, B. K. J. Org. Chem. 1951, 16, 1736–1740.
11. Cabiddu, S.; Maccioni, A.; Secci, M. Gazz. Chim. Ital. 1969, 99, 397–410.
12. Cabiddu, S.; Maccioni, A.; Secci, M. Gazz. Chim. Ital. 1969, 99, 1095–1106.
13. Clark, J. H.; Holland, H. L.; Miller, J. M. Tetrahedron Lett. 1976, 3361–3364.
14. Dallacker, F.; Morcinek, R.; Rabie, A. Z. Naturforsch. Sect. B 1979, 34, 1434–1436.
15. Castillo, P.; Rodriguez-Ubis, J. C. Synthesis 1986, 839–840.
16. Bashall, A. P.; Collins, J. F. Tetrahedron Lett. 1975, 3489–3490.
17. Cabiddu, S.; Maccioni, A.; Secci, M. Synthesis 1976, 797–798.
18. Zelle, R. E.; McClellan, W. J. Tetrahedron Lett. 1991, 32, 2461–2464.
19. Lee, H. H.; Denny, W. A. J. Chem. Soc., Perkin Trans. 1 1990, 1071–1074.
20. Cambie, R. C.; Janssen, S. J.; Rutledge, P. S.; Woodgate, P. D. J. Organomet. Chem. 1991, 420, 387–418.
21. Bonthrone, W.; Cornforth, J. W. J. Chem. Soc. (C) 1969, 1202–1204.
22. Archer, A. W.; Claret, P. A.; Hayman, D. F. J. Chem. Soc. (B) 1971, 1231–1240.
23. Loriot, M.; Robin, J. P.; Brown, E. Tetrahedron 1984, 40, 2529–2535.
24. Sarma, J. A. R. P.; Nagaraju, A.; Majumdar, K. K.; Sammel, P. M.; Das, I.; Roy, S.; Mcghie, A. J. J. Chem. Soc., Perkin Trans. 2 2000, 6, 1119–1124.
25. Cabiddu, S.; Fattuoni, C.; Floris, C.; Gelli, G. Heterocycles 1988, 27, 1679–1684.
Source: Tetrahedron 59 (2003) 4383-4387
DOI:10.1016/S0040-4020(03)00619-7
The faster you run, the quicker you die.
(Chief Bee)
02-05-04 08:34
No 486519
(Rated as: excellent)
A Convenient, High-Yielding Method for the Methylenation of Catechols
Bashall, A.P.; Collins, J.F.
Tetrahedron Letters 3489-3490 (1975) (../rhodium /methyl
____ ___ __ _
A Simple, High-Yielding Method for the Methylenation of Catechols
R.E. Zelle & W.J. McClellan
Tetrahedron Letters 32(22) 2461-2464 (1991) (../rhodium /methyl
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
05-22-04 13:51
No 508760
(Rated as: good read)
The Methylenation of Catechols
W. Bonthrone and J. W. Cornforth
J. Chem. Soc. (C), 1202-1204 (1969) (../rhodium /methyl
Abstract
High yields in the methylenation of catechols by methylene chloride are obtained by song a polar aprotic solvent for reaction and maintaining low concentrations of the catechol dianion.
____ ___ __ _
Hydrogen Bonding in Organic Synthesis. IV.
A Simple, High-Yield Methylenation of Catechols
J.H. Clark. H.L. Holland, J.M. Miller
Tetrahedron Letters 3361-3364 (1976) (../rhodium /methyl
Abstract
We wish to report a simple method for the high-yield methylenation of catechols which requires neither the use of strong base nor any special precautions such as a nitrogen atmosphere or controlled addition of reagents. The reaction of a catechol with dihalogenomethane in DMF in the presence of an excess of potassium or caesium fluoride provides a high yield of the corresponding methylenedioxy compound in a relatively short period of time.
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
05-27-04 19:38
No 509950
(Rated as: good read)
These articles by the russian chemist E.D. Laskina explores the use of methylene chloride in the methylenation of catechols, which due to its low cost makes it very attractive for large-scale reactions. Slow addition of the methylene chloride to the reaction mixture in ethylene glycol heated to ~125°C, as well as continously returning what has evaporated to the reaction mixture through an "inverted Dean-Stark trap" makes it possible to perform the reaction with methylene chloride without the use of pressure. The preparation of methylenedioxybenzene (1,3-benzodioxole) from pyrocatechol is described, as well as ortho-Safrole from 3-allylpyrocatechol (2,3-dihydroxyallylbenzene), both preparations yilding about 50% of the desired product after purification.
Certain Reactions of Methylene Chloride Conducted in High-Boiling Solvents Without use of Pressure. Part 1.
E. D. Laskina
J. Appl. Chem. USSR (Engl. Trans.) 32, 895-899 (1959) (../rhodium /methyl
____ ___ __ _
Certain Reactions of Methylene Chloride Conducted in High-Boiling Solvents Without use of Pressure. Part 2.
E. D, Laskina and T. A. Devitskaya
J. Appl. Chem. USSR (Engl. Trans.) 34, 2214-2216 (1961) (../rhodium /methyl
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
06-06-04 23:48
No 511836
(Rated as: excellent)
Cupric Oxide as an Efficient Catalyst in Methylenation of Catechols
Masao Tomita and Yoshiaki Aoyagi
Chem. Pharm. Bull. 16(3) 523-526 (1968) (../rhodium /methyl
Abstract
Methylenation of catechols with methylene halides was found to be catalyzed more effectively by cupric oxide in dimethylformamide.
____ ___ __ _
The Synthesis of 3,4-Dihydroxy-2-Methoxybenzaldehyde: The use of Methylenedioxy as a Protecting Group
I. R. C. Bick and R. A. Russell
Aust. J. Chem. 22, 1563-1568 (1969) (../rhodium/pdf /2-meo-pipero
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
11-13-04 06:13
No 541376
Preparation and Properties of Naphtho[1,2-d]- and Naphtho[2,3-d]-1,3-dioxolen
Franz Dallacker, Ralf Morcinek und Arafat Rabic
Z. Naturforsch. 34b, 1434–1442 (1979) (../rhodium/pdf /dallacker.md
Summary
We describe the preparation of unsubstituted 1a, of 4-methoxy- (1b), and of 5-methoxynaphtho[1,2-d]-1,3-dioxole (1c). Treatment of 1a with bromine leads to 5-bromo-naphtho-[1,2-d]-1,3-dioxole (1d). The carboxylic acid 1f and 5-methyl-thio-naphtho[1,2-d]-1,3-dioxole
Because of less SE-reactivity of 1c the carboxylic acid 1l and methyl-thio-naphtho[1,2-d]-1,3-dioxole (1n) can only be prepared from the lithium-compound as intermediate. Naphtho[2,3-d]-1,3-dioxole (2a) can easily undergo electrophilic substitution, showed by nitration yielding 2b, acetylation yielding 2c, bromination yielding 2d and 2e. The carboxylic acid 2j, the methyl-derivative 2k, the thionaphthole 2l, and the aldehyde 2m are prepared from the lithium-compound of 2d.
Treating 2e with n-butyl-lithium leads to the dilithium-naphthyl derivative, a suitable starting material to obtain the dimethyl-compound 2n, the dicarboxylic acid 2o, and the dialdehyde 2p. Monomethyl-thio- (2q) and dimethyl-thio-naphtho[2,3-d]-1,3-dioxole
The Hive - Clandestine Chemists Without Borders