02-26-01 04:17
No 175553
A couple of days ago, i was bored so i plugged a sidearm flask containing a spoonful of L-tryptophan; applied a fridge-pump vacuum (+- 30mmHg) and heated on an asbestos plate above a burner flame.
Evolution of gas (CO2) started quickly, the shit turned red and after trying to crystallize the goo with some EtOH, it showed clearly prismatic crystals, but it all oiled out.
But hey, this was just to try.
Tonight , after work, i will try to suspend/dissolve tryp in ethylene glycol with a trace of ketone added (acetone) and hold it at 150°C for a couple of hours,
more on this later...
comments, criticism ?
e109
(Moderator)
02-26-01 10:54
No 175601
Maybe it's a bad idea to use glycol with its alcoholic OH groups. They are competing with the tryptamine NH2 group in forming the reactive imine species from the ketone.
The acetone is a very low boiling solvent which may disappear before its reaction.
Good luck.
(Hive Bee / Eraser)
02-27-01 04:07
No 175726
Yes, i've considered that before. But it's a common solvent with a high bp. (somewhere in the 190°C's), so why wouldn't i give it a try. Does cyclohexanol's OH group give the same problems? Yet it gives very good yields.
You don't really need a ketone catalyst too, i 've got tryptamine from heating tryp under fridge pump vacuum, until CO2 evolution begins (shit turns orange and melts a bit, while bubbling vigorously). Resulting goo is washed twice with a sat'd NaHCO3-sol, filtered, and dried.
Next thing will be cleaning the red crystals by sublimation.
How about using a higher boiling ketone from natural sources,
like isopinocamphone from Oil of Hyssope ?
Want i'm trying to do is to find a good yielding consistent way to decarboxylate, and i'm willing to test on smaller (gram) scale:
I) Different solvents, ketones and T°
II) Decarboxylation of tryp under vacuum with a catalyst (eg. barium oxide)
I have most things lying around and am tryp-unlimited (to a certain extend) so i will report my results here every time, so i expect your ideas, comments and criticism on this, bees.
later,...
e109
(Hive Addict)
02-27-01 11:14
No 175783
Try cetyl alcohol, it is a common cosmetic chemical.
Seems to have idea properties.
The cosmetic version has bp(195-200C at 50mm Hg) so its high boiling. Says its a liquid at room temp, probably highly viscous though.
It is immicible with water which will allow the Tryptamine HCl to be easily isolated. It is miscible with most common organic solvents which makes washing the aqueaous trypt HCl easy.
I would suggest a higher boiling keytone than acetone, either MEK(easily avail otc) its still low boiling however but a little better than acetone.
Or MDP2P could be used(see rhodiums site for its synth)
Do Your Part To Win The War
(Hive Bee)
03-11-01 06:11
No 177879
Um, are you sure about that??!! How would an alcohol compete with an amine to form a Schiff base with a ketone? Maybe I'm missing something, but somehow that doesn't make any sense to me...
Please elaborate,
-me
(Moderator)
03-13-01 00:30
No 178183
Alcohols react with ketones to (hemi-) acetals. And the di-alcohol glycol forms a stable cyclic acetal.
(Hive Bee)
03-13-01 21:15
No 178363
Lilienthal,
Okay, the reaction of glycols forming dioxolanes, not imines, competes with the desried schiff base formation reaction - that makes sense to me.
That was the misunderstanding. I thought you were suggesting ketones and glycols forming imines, which obviously doesn't make sense. Naturally you weren't, so nevermind.
Good point about acetal formation, I must say. Usually, one uses a acid catalyst to promote dioxolane formation, with tosylate being a particularily fine choice, but who's to say its needed at those temperatures?
I wouldn't worry about alcohols posessing only one hydroxyl group forming acetals though - especially cyclohexanol. Generally, you need a fairly decent dehydrating agent or some equivalent scheme to make such a thing happen. Besides, there'd be some serious steric hindrance issues making that less than probable.
(Kitty-KrZ / Eraser)
04-02-01 12:21
No 181680
According to Drone, acetone, DMSO and tryptophan, when heated together, provides tryptamine in good yield. I KNOW I have the Old Hive thread in which he states this printed out somewhere and as soon as I find it, I'll post the details.
--PK
(Hive Addict)
05-01-01 17:05
No 188461
psycho kitty
How did drone propose one would get the tryptamine out of the DMSO?
Seems kind of messy becasue dmso is soluable in most common solvents and water.
Evaporate under reduced pressure?
Do Your Part To Win The War
(Hive Bee)
05-01-01 22:04
No 188518
I put tryptophan in the microwave with simply water and let it reflux on defrost for about ten minutes. In the end what I got was a orange yellow substance that smelled like mothballs. I then ate it
My reflux appy was simple. I put a plate over the bowl the tryptophan was in. Every few minutes I would replace some of the water that would leave the reaction. Something happend because it changed colors but what changed is a different story. I know though that they make pressure cookers for microwaves.
(Hive Addict)
05-02-01 03:48
No 188595
LOL
Thats some funny shit sphybrid
Do Your Part To Win The War
(Hive Bee)
05-02-01 19:24
No 188787
Point is ethylene glycol may work in the microwave since that is a common solvent for the microwave rxns. Isolating the trytamine shouldn't be that hard. Vacu suck methods and heat should suffice with recrystallization from proper solvent. I think the microwave for this reaction would be premo. If I put some more thought into it i know I could figure it out:D
(Hive Bee / Eraser)
05-10-01 08:42
No 193271
I was away for some time , but this is what meanwhile happened:
~2 grams of L-tryptophan was dissolved in a small quantity of ethylene glycol, the technical shit. 4 drops of acetone were added and the mixture was refluxed on an oil bath for an hour.
The mixture was poured in a beaker and allowed to cool to RT. Then the beaker was put in the freezer and was left there for 2 days.
A layer of WHITE, fluffy crystals precipitated, it was a small gram, after washing 2x with conc Na2CO3-sol and 1x with dH2O. It has a slight smell.
I have yet have to take a mp reading to confirm .
If this could be tryptamine, things could look VERY well>
Don't try to dialkylate or do something to dirty reddish stuff, it will result in total frustation and brown end products.
Maybe we've got something here ladies and gentlemen...
e109
(Hive Bee)
05-10-01 10:47
No 193292
You could have used MEK also since it has a higher BP. I am looking for something I can use in the mic. I know it tranformed some of my product to the coresponding tryptamine. Large amounts of CO2 where given off.
(Moderator)
05-10-01 11:15
No 193295
Interesting method to recrystallize tryptophan
(Moderator)
05-10-01 11:18
No 193296
What I tried to say was: tryptamine should be highly soluble in ethylene glycol, the precipitate is possibly unchanged tryptophan
(Hive Addict)
05-10-01 17:49
No 193396
Sphybrid
How do you know that CO2 was given off???
Was your glycol dryed??? It is hygroscopic and will pick up water which would boil off in the microwave giveing the illusion of CO2
Do Your Part To Win The War
(Hive Bee)
05-10-01 19:36
No 193422
Beilstein says that it can be done in diphenyl ether to 63% yields. It simply said tryptophan was heated for 10 hours in diphenyl ether(I would assume under reflux). Maybe if they refluxed longer yields would have been higher??????
(Hive Bee / Eraser)
05-14-01 04:09
No 194265
I'm sorry boys 'n girls , but Lilienthal is right: these are surely some nice tryptophan crystals ,
b'cause tryptamine melts at 115-117°C, and this shit stays solid when going over 135°C.
But lets keep experimenting; foxy, the cetyl alcohol (where i live) is a solid at RT, but i thought cyclohexanol is also , and we KNOW this gave good results.
I'm gonna look up the original papers because i want to know more about the solvent/ketone mechanism,
e109
(Hive Addict)
05-14-01 06:09
No 194289
Who cares if its a solid at room temp the reaction requires heat!!!
I am planning on trying this with naphthalene and cyclohexene soon. A Hot A/B should get rid of the naphthalene.
Do Your Part To Win The War
(Hive Bee)
05-14-01 09:55
No 194318
Napthalene will dissolve pretty good in DCM. And you certainly don't want to add DCM to a more than hand-warm water solution.
(been there, done that, decorated the ceiling again.)
(Hive Bee)
05-14-01 20:15
No 194487
Beilstein says that it can be done in diphenyl ether to 63% yields. It simply said tryptophan was heated for 10 hours in diphenyl ether(I would assume under reflux). Maybe if they refluxed longer yields would have been higher??????
With the above reaction one can seperate the two recover the tryptophan and do it again seperate recover and do it again etc....{The point is that the use of a ketone was not used so it isn't always needed aparently} Polyethylene glycol is a polyether with alcoholic ends. Might work, might not. CHeck er, out!
With a simple column you should be able to seperate these two quite easily(tryptophan from tryptamine). I also did not find much on the use of ethylacetate to do the seperation. That could prove to be quite useful also. On one hand the decarboxylated product could be much more soluble in the EtOAc than the carboxylated precurser.
Diphenyl ether has a very electron poor Oxygen linkage though which could be better than a glycolic polyether in this reaction.
Sorry best I can do!
(Hive Bee)
05-15-01 00:38
No 194538
PEG, yeah, just what I was thinking. With fewer hydroxyls, it won't bump into Lilienthal's objection as badly as glycol, or say, glycerine would. Naphthalene, hm, who knows -- with this "solvent", how could you ever get your Tryp out? Sounds like you're in a worse fix than with DMSO! Any way a vegetable cooking oil could dissolve tryptophan? Aqueous acid would sure pull the basic tryptamine out of that, a lot easier than it would the amphoteric amino acid. But for a catalyst, try benzoquinone! Betcha that would work.
Turning science fact into <<science fiction>>
(Hive Addict)
05-15-01 14:17
No 194676
Naphthalene shouldn't be so hard to remove. An A/B at 90°C should be all liquid and remove most of the naphthalene. The remainder can be easily washed out with DCM(of course not at 90°C).
Do Your Part To Win The War
(Moderator)
05-15-01 14:33
No 194677
You don't have to heat for the acid / base extraction if you use an organic solvent
(Hive Addict)
05-15-01 15:53
No 194697
Lili
The melting point of naphthalene is approximately 80°C so doing the initial acidic water extraction at 90°C will bee necessary.
Do Your Part To Win The War
(Moderator)
05-15-01 15:59
No 194700
Use an organic solvent during the acidic extraction. Naphtalene will stay in the organic phase, the tryptamine salt moves into the aqueous phase.
(Hive Addict)
05-15-01 16:05
No 194704
Yea ok as usual someone comes along with a better method
Thanks Lili
Do Your Part To Win The War
(Hive Bee)
10-04-01 15:14
No 220393
psychokitty> "According to Drone, acetone, DMSO and tryptophan, when heated together, provides tryptamine in good yield. I KNOW I have the Old Hive thread in which he states this printed out somewhere and as soon as I find it, I'll post the details."
So, does this method work or not? I got a bit lost in all the comments. I need to make some tryptamine soon.
Maighstir.
Vae victis meretrix est et mors venit.
(Moderator)
10-04-01 16:12
No 220425
My (purely theoretic) guess is that it may work under absolutely anhydrous conditions with removing the water formed during imine formation. Then (and only then) it wouldn't matter if all your acetone boils off after a few minutes of heating.
But I would simply use a much higher boiling ketone.
(Old P2P Cook)
10-04-01 20:52
No 220513
Rhodium just recently posted in this Forum a very promising looking method using copper ion to promote the decarboxylation. Post 211603 (Rhodium: "Another tryptophan decarboxylation", Tryptamine Chemistry)
(Distinctive Doe)
10-05-01 01:16
No 220598
Try THUJONE as the ketone, just get some high thujone wormwood essential oil. I think it might work.
THUJONE
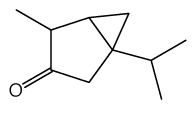
Do Your Part To Win The War
(Stranger)
10-05-01 14:16
No 220709
artemisia absinthium is around 50% thujone
thuja plicata essential oil is around 90% thujone
(Master Whacker)
10-06-01 16:22
No 221031
The DMSO/copper chelate decarboxylation works like a charm with phenylalanine. SWIM has found that yields are in the 70% range with this amino acid. Unlike my cyclohexanone/cyclohexanol decarboxylation archived at Rhodiums site, this procedure produces repeatable yields.
Make sure decarboxylation is carried out in a hood as DMSO vapors are horrible!
(Hive Bee)
10-08-01 09:47
No 221692
Ritter, do the yields of your reaction fluctuate? I always thought it was a high-hitter (80+%), and the DMSO reaction gives only 40% yield.
First i wanted to try the DMSO reaction, but comparing the prices of DMSO and cyclohexanol, think SWIM's going to try a chelate in cyclohexanol reaction, next month or so.
It's always good to try a new idea, isn't it?
e109