06-11-02 23:06
No 320083

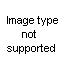
What can be done with this to get to the ever lovly DMT, and which would be better?
(Hive Prodigy)
06-12-02 00:47
No 320111
The first chemical is far superior. In fact, IMHO the second is very near worthless.
The first could be a precursor to multiple tryptamines, because that nitrile function is able to undergo several transformations in different reactions.
For DMT, you could hydrogenate it to tryptamine, and methylate it to the quaternary ammonium iodide with methyl iodide, and react it with dimethylamine to form DMT, trimethylamine and the iodide salt of the base used to promote the reaction, such as KO-tBu would yield t-BuOH + KI.
Overall: If indole = R, then
RCH2-C#N ==NaBH4==> R-CH2-CH2-NH2
R-CH2-CH2-NH2 + 3MeI --> R-CH2-CH2-N+(-Me)3(I-) + 2HI
R-CH2-CH2-N+(-Me)3(I-) + HNMe2 + KO-tBu --> R-CH2-CH2-NMe2 + NMe3 + t-BuOH + KI
IMO the second compound's lacking of the second carbon in that chain makes it an incredible bitch to get something useful out of it. Do nitriles act as leaving groups at all? Ive never seen an example of them being a leaving group. That's the only thing I can think of that isn't long and ridiculous, really. You would need a reaction that just outright replaced the nitrile with something more useful. I personally know of no such reaction (yet) but maybe others do.
PrimoPyro
(Hive Addict)
06-12-02 01:12
No 320120
just an idea: make out of the 1st one subst. AMTs
like (meth)amphetamine from BzCl:
1. R-CH2-CN + AcEt ---(NaOEt)---> R-C(CN)-CO-CH3
2. R-CH(CN)-CO-CH3 ---(much H2SO4 + heat)---> R-CH2-CO-CH3
3. R-CH2-CO-CH3 + NH2R ---(reductive amination)---> R-CH2-CH(CH3)-NHR
4. R-CH2-CH(CH3)-NHR + R'-I ---> R-CH2-CH(CH3)-NRR'
2. and 3. won't work, right?
(Hive Prodigy)
06-12-02 02:28
No 320135
2 should work, look at the cleavage of the CN to make phenylacetic acid on Rhodium's phenylacetone document.
Mandelic acid methods operate on this type cleavage. Or you can CTH it with limonene to reduce the nitrile to a methyl.
3 will work up until the methylation part. There used to be a sticky thread in this forum by Lili called, "You cant dimethylate tryptamines with Me2SO4 or MeI!!" haha.
It quaternizes the amine is why. So direct methylation wouldnt work, but I think you can reductively aminate the ketone with dimethylamine, since the intermediate is the aminol RNH-COH --> RN=C + H2O so maybe it will work with diethylamine.
(Hive Addict)
06-12-02 03:16
No 320168
> 2 should work, look at the cleavage of the CN to make
> phenylacetic acid on Rhodium's phenylacetone document.
yes, it's the "org.syn." route to P2P. i was just wondering
if the molecule survives such harsh conditions.
> 3 will work up until the methylation part.
ah! but where did you see a methylation? the idea was to
reductively aminate with MeNH2 to get n-methyl-AMT or
with NH3 to get AMT. but didn't Lili say that acid &
reducing conditions is a big no-no?
if it works, you could then alkylate with a R > Me. (i
wrote R on purpose
> but I think you can reductively aminate the ketone with dimethylamine.
can you? i thought when reacting a secondary amine with
a ketone, you get an enamine (NRR-C=). can that be easily reduced too?
(Hive Prodigy)
06-12-02 03:19
No 320170
You do get an enamine. If you were to use something like NaBH4 I would think it would reduce the -ene portion just dandy. But will it reduce the indole to an indoline? I have no idea, Im still new to tryp chem.
You lost me with the writing R on purpose thing.
(Hive Addict)
06-12-02 04:04
No 320190
> But will it reduce the indole to an indoline?
hm... the standard route to DxTs is with LAH.
shulgin makes AMT over the nitropropene and reduces with LAH.
so i guess no? i too don't know what goes and what doesn't
with tryptamines...
(Newbee)
06-12-02 14:11
No 320417
Nitrostyrenes reduce easily with palladium/C under mild conditions... my aunt tells me that it is an easier work up then with borohydide or LAH!
I dunno, but I been told ... You never slow down, you never grow old!
(Chief Bee)
06-12-02 15:35
No 320453
Nitrostyrenes reduce easily with palladium/C under mild conditions...
Could you specify this, preferably very detailed, in a post in the Methods forum (it is off topic here)?
(Hive Bee)
06-15-02 22:18
No 321966
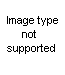
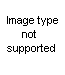
I dont take drugs to be happy...
I do because they remind me that I am happy
(Chief Bee)
06-16-02 01:31
No 322011
The first one seems not too useful (no 9,10 double bond, and a methyl group on the indolic nitrogen).
The second looks like isolysergol to me (the primary alcohol, which upon oxidation would give isolysergic acid). The "iso" part is the double bond in the D ring being in the wrong place, it should be one step further towards the indole benzene ring.
I believe there is a procedure for iso-lysergic acid to lysergic acid somewhere on my page, either in MV Smith's "Psychedelic Chemistry" or Professor Buzz' "Recreational Drugs".
(Stranger)
06-16-02 08:27
No 322081
Rh,
Isolysergic acid referres to the 8-position stereoisomer of lysergic acid. Lysergic acid is (S)-isomer, and iso-lysergic acid is the (R)-isomer.
Yes, you can epimerize lysergic acid and isolysergic acid, since under slightly acidic conditions the carbonyl will undergo a reversible reaction to form the corresponding achiral enol. When the enol reverts to a carbonyl, it may form either the (S)- or the (R)-isomer, but lysergic acid is the more favorable of the two stereoisomers, due to some steric hindrace issues.
However, and I hate to say it, there is just no way you're going to get that double bond to move from the 8,9-position to the 9,10. The problem is that by doing so, you're adding a huge amount of thermodynamically unfavorable ring strain. Go ahead and make a model to try it out: with the 8,9- there's some flexibility in the non-aromatized rings, but by placing the double bond on the 9,10-position, you're forcing the 5, 8, 9, and 10 carbon atoms to basically lie in the same plane as the indole ring - thus eliminating any hope for the two 6-membered non-aromatic rings of conforming to anything resembing a "comfy" chair-configuration. This is one of the reasons why the 9,10-double bond is so prone to attack - adding across it relieves a huge strain on the molecule.
As a visual aide, here are lysergic acid and isolyserigc acid.
lysergic acid:
Molecule: lysergic acid ("[H][C@@]12Cc3c[nH]c4cccc(C1=C[C@@H](C(
isolysergic acid:
Molecule: isolysergic acid ("[H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](C(=
(Chief Bee)
06-16-02 10:10
No 322099
Thanks for the clarification! I found a little more of this in Tihkal:
LSD is an unusually fragile molecule and some comments are in order as to its stability and storage. As a salt, in water, cold, and free from air and light exposure, it is stable indefinitely. There are two sensitive aspects of its structure. The position of the carboxamide attachment, the 8-position, is affected by basic, or high pH, conditions. Through a process called epimerization, this position can scramble, producing isolysergic acid diethylamide, or iso-LSD. This product is biologically inactive, and represents a loss of a proportionate amount of active product. A second and separate point of instability is the double bond that lies between this 8-position and the aromatic ring. Water or alcohol can add to this site, especially in the presence of light (sunlight with its ultraviolet energy is notoriously bad) to form a product that has been called lumi-LSD, which is totally inactive in man. Oh yes, and often overlooked, there may be only an infinitesimal amount of chlorine in treated tap water, but then there is only an infinitesimal amount of LSD in a typical LSD solution. And since chlorine will destroy LSD on contact, the dissolving of LSD in tap water is not appropriate.
(Hive Prodigy)
06-16-02 10:32
No 322103
Im not saying it is high yielding, but this isomerization of this particular double bond, from the 8,9 position to the 9,10 position, has been done before, in Oppolzer's synthesis of lysergic acid.
After the intramolecular Diels-Alder reaction, closing the C and D rings, the double bond is located in the 8,9 position, and the molecule is isomerized in a future step to lysergic acid.
Paspalic acid also contains the double bond in this location and needs isomerization. Paspalic acid --> lysergic acid has been documented and brought up here as well.
PrimoPyro
(Moderator)
06-16-02 11:19
No 322130
It looks like the double bond feels pretty good in conjugation with the aromatic ring, ignoring the ring strains...
(Stranger)
06-16-02 16:36
No 322230
Indeed, I'm sure the double bond feels quite comfortable conjugated with the indole ring, ignoring the ring strains.
Thank goodness for the Hive. This discussion began me thinking more about this paspalic->lysergic business. Yes, there is a great deal of added strain to the molecule, but at the same time, a bond in the 9,10-position would be conjugated with the indole ring, which is certainly an energy incentive. So, if one could find a mechanism by which the reaction could happen (arguments about sterics aside), then one would simply need to find conditions where the conjugation of the double bond would overrule the disadvantages of ring strain...
So, I did a quick literature search, and hit gold.
In 2001, inventor Jean-Claude Gallier, working for Aventis Pharma S.A. of France patented a method of isomerizing paspalic acid into lysergic acid using tetraalkylammonium hydroxide (organic-soluble bases) as the catalyst. The application number is 2001002395, 11 Jan 2001, which corresponds to Patent WO0102395, FR2795728, EP1196415.
This reaction wasn't available through Beilstein Crossfire, and If it weren't for the sort of idea exchange here at the Hive, I wouldn't have felt any need to question what I had been taught in school a few years ago. I would have never pursued this idea any further without people here inquiring as they did. This was wonderful.
(Stranger)
06-29-02 15:33
No 326955
Ever since I learned that paspalic acid could be isomerized into lysergic acid, I've been reexamining what I've thought about ergot chemistry. In particular, I've been thinking about hydergine and other 9,10-dihydrolysergic acid derivatives.
Hydrogenated lysergic acid derivatives are nowhere near as closely regulated as the non-hydrogenated ones. The reason for this is that 9,10-dihydrolysergamides are nowhere near as active or interesting.
But...
What if dehydrogenation really is possible? Sure, H2 isn't going to split off by itself spontaneously, but maybe under the right conditions there is a reaction, or a series of reactions, that would allow this to happen.
I know that DDQ has been used to dehydrogenate benzocyclohexane systems to afford a double bond that is conjugated with the benzene ring, but would it work here? This sort of reaction has been used rather extensively in recent terpenoid syntheses, but how will that pyrrole ring affect it?
Here's a general example of this reaction:
Molecule: dehydrogenation1 ("[H][C@]24CC[C@]1(C)[C@@H]([R])CC[C@@]1
Here are some articles related to this:
Tetrahedron Letters, (2002), 41(11), 1729-1731.
Comptes Rendus de l¤Academie des Sciences, Serie IIc: Chimie, (2001), 4(3), 201-205.
J. Chem. Soc., Perkin Trans.1 (1955), (22), 2813-15
Steroids, (1995), 60(12), 809-11.
Recl. Trav. Chim. Pays-Bas, (1993), 112(12), 627-34.
Synlett, (1992), (10), 821-2.
Patent US4882319
Helv. Chim. Acta, (1989), 72(4), 725-30.
Another route might be to take it in two steps: allowing dihydrolysergamides to react with LDA and I2 should add an iodide beta to the carbonyl. If this were followed up with an E2 elimination reaction, you'd have successfully dehydrogenated your starting material.
I don't know. I'm throwing out guesses right now. Does anybody else have another approach to this problem?
(Moderator)
06-29-02 17:07
No 326975
I haven't read your refs (yet), but I remember that lysergic acid derivatives rearrange under dehydogenating conditions into their energetically even more favorable naphtalene derivatives
(Stranger)
06-30-02 13:40
No 327279
Difficult to control the dehydrogenation, you say? This is interesting information. You wouldn't be able to cite any references for the conversion of lysergic acid derivatives into corresponding napthalenes, do you? Which specific conditions are these? Has anything been done with DDQ on the ergoloid nucleus, or has it all been Pt or Pd catalyzed dehydrogenations? I think knowing what conditions to avoid is certainly a very important thing to know.
This is why I keep thinking about halogenation of either the 8-, 9- or 10-position. The 9- or the 10-position would be ideal, though I'm not sure what reagent one could use to afford this. The 8-position might be alright, but the challenge will then be to make the E2 reaction form a 8,9-double bond (paspalic acid), rather than a 7,8-double bond (bollocks.)
Hmm, are there any other leaving groups that could be added that would allow for a desirable E2 elimination reaction? Interesting...