09-25-02 04:38
No 360199
Is there a working synthesis for 5meo-AMT? I UTFSE, and searched Tihkal, as well as used various search engines. Or could someone point me in the direction of a similar synthesis, or a synthesis with similar reaction conditions I could see.
Secondarily is there an easy route from AMT to 5meo-amt, or a similar article, or entry that I could see regarding this proceedure.
Thank you.
(Chief Bee)
09-25-02 04:50
No 360204
You cannot make 5-MeO-AMT from AMT unfortunately.
5-MeO-AMT Synthesis: http://www.erowid.org/library/books_onli
(Stranger)
10-06-02 19:56
No 365077
You could treat AMT with Ac2O, which would acetylate the two nitrogens. With the acetyl groups in place, you could treat it with bromine to selectively brominate the 5-position. Following that, nucleophilic aromatic substitution using excess NaOMe and a Cu(I)-species as a catalyst will methoxylate the 5-position. Saponification using KOH should give you 5-MeOAMT. This seems to me to be the most plausible route between the two, though I think you'd be better off using some other starting material than AMT.
(Moderator)
10-07-02 09:20
No 365373
The bromine will probably go into the indole-2-position
(Stranger)
10-09-02 00:13
No 366210
That's hardly the biggest obstacle to overcome, now is it? Treat the indole with sodium bisulfite to reversibly protect the 2-position, and then remove it later on by treating it with base.
(Chief Bee)
10-09-02 02:02
No 366250
dmitri: Do you have any references which discusses the use of bisulfite as a indole-2-protecting group, with scope and limitations?
(Stranger)
10-09-02 08:26
No 366378
Rh,
There are a couple of articles that come to mind immediately, though I'm pretty sure you have them already. In fact, these two have gotten a fair amount of attention here at The Hive over the years. The first one is a paper that claims to describe a successful route to 5-bromoindole using this method, but I gaurantee you that it won't work. The second is a peer-reviewed revision of the previous method, with some crucial improvements, including a lot of important practical tips and a discussion of the theory behind them.
Chem.Ber. 1962, 95, 2205.
Org.Prep.Proc.Int. 1985, 17(6), 391.
Two articles that focus more closely on the mechanism and application of bisulfite addition to indoles under acidic conditions are the following:
Ber. 1925, 58, 1520.
Angew.Chem. 1957, 69, 727.
I know there's more written on this topic, but I'm away from my article collection right now, so I can't give you any more references. I'll send more information when I can...
(Chief Bee)
10-09-02 08:39
No 366381
Great! I have a theoretical pet project of making the bisulfite addition compound of tryptamine, and then subject it to some kind of high-yielding reductive methylation such as an eschweiler-clarke to make 2-sulfonyl-DMT, then deprotect to give DMT in high yield. As you know, the methylation cannot be performed without protecting group, as pictet-spengler side reactions predominate (except in the case of using methanolic formaldehyde with NaBH3CN under neutral conditions, where moderate yields are possible depending on substrate).
(Hive Bee)
10-10-02 00:57
No 366635
Here we have a great example of the vast amount of information that has been archived by Rhodium. Even this protection is described in ../rhodium /5-br-in
Great!
(Stranger)
10-13-02 20:34
No 368060
The one other example in the literature that uses this 2,3-bisulfite addition protection scheme on indoles that's worth mentioning is Bioorg. Med. Chem. Letters, 11, 793-795 (2001). This is a fine article by the good Dr. D.E. Nichols on some novel fluorinated tryptamines, and it is definately worth a trip to the library. I just thought I'd mention it for the sake of thoroughness.
(Chief Bee)
10-14-02 00:47
No 368147
(Rated as: excellent)
Effect of Ring Fluorination on the Pharmacology of Hallucinogenic Tryptamines
Joseph B. Blair, Deborah Kurrasch-Orbaugh, Danuta Marona-Lewicka, Medhane G. Cumbay, Val J. Watts, Eric L. Barker, and David E. Nichols
J. Med. Chem. 2000, 43, 4701-4710 (2000) (../rhodium/pdf /nichols/nich
Abstract
A series of fluorinated analogues of the hallucinogenic tryptamines N,N-diethyltryptamine (DET), 4-hydroxy-N,N-dimethyltryptamine (4-OH-DMT, psilocin), and 5-methoxy-DMT was synthesized to investigate possible explanations for the inactivity of 6-fluoro-DET as a hallucinogen and to determine the effects of fluorination on the molecular recognition and activation of these compounds at serotonin receptor subtypes. The target compounds were evaluated using in vivo behavioral assays for hallucinogen-like and 5-HT1A agonist activity and in vitro radioligand competition assays for their affinity at 5-HT2A, 5-HT2C, and 5-HT1A receptor sites. Functional activity at the 5-HT2A receptor was determined for all compounds. In addition, for some compounds functional activity was determined at the 5-HT1A receptor. Hallucinogen-like activity, evaluated in the two-lever drug discrimination paradigm using LSDtrained rats, was attenuated or abolished for all of the fluorinated analogues. One of the tryptamines, 4-fluoro-5-methoxy-DMT (6), displayed high 5-HT1A agonist activity, with potency greater than that of the 5-HT1A agonist 8-OH-DPAT. The ED50 of 6 in the two-lever drug discrimination paradigm using rats trained to discriminate the 5-HT1A agonist LY293284 was 0.17 µmol/kg, and the Ki at [3H]8-OH-DPAT-labeled 5-HT1A receptors was 0.23 nM. The results indicate that fluorination of hallucinogenic tryptamines generally has little effect on 5-HT2A/2C receptor affinity or intrinsic activity. Affinity at the 5-HT1A receptor was reduced, however, in all but one example, and all of the compounds tested were full agonists but with reduced functional potency at this serotonin receptor subtype. The one notable exception was 4-fluoro-5-methoxy-DMT (6), which had markedly enhanced 5-HT1A receptor affinity and functional potency. Although it is generally considered that hallucinogenic activity results from 5-HT2A receptor activation, the present results suggest a possible role for involvement of the 5-HT1A receptor with tryptamines.
(Chief Bee)
02-01-03 02:57
No 403143
(Rated as: excellent)
Suggested procedure:
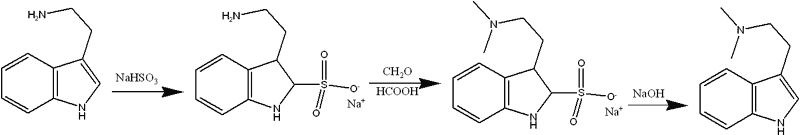
Beiträge zur Chemie des Indols, XIII.
Eine Neue Synthese 5- und 7-substituierter Indole
J. Thesing, G. Semler, G. Mohr
Chem. Ber. 95, 2205-2211 (1962) (../rhodium/djvu /indole-2-bi
Dmitri: You said the above article was bunk... Why?
 (10-21-04): Related post: Post 478255 (Vitus_Verdegast: "Preparation of 5- and 7-substituted indoles", Tryptamine Chemistry)
(10-21-04): Related post: Post 478255 (Vitus_Verdegast: "Preparation of 5- and 7-substituted indoles", Tryptamine Chemistry)
(Moderator)
02-02-03 13:34
No 403579
A problem could be indoline-1-NH alkylation cause it's a simple amine (aniline).
(Chief Bee)
02-02-03 14:36
No 403586
Are you aware of any selective protection, which wouldn't affect the aliphatic amine? It would be sad if this excellent idea was to be found unusable after all...
(Hive Bee)
02-06-03 09:52
No 405014
I understand the cursiousity and all. But whay don't you just buy some? It'll save you tons of money and time (unless of course like I said your just curious)?
(Chief Bee)
02-06-03 11:07
No 405043
Buy what? If you ask "why not buy the DMT" then you have missed my point. I am not looking for a way of aquiring DMT, I am looking for a method of protecting the indole ring of tryptamine so that it can be reductively alkylated using standard Eschweiler-Clarke conditions (formaldehyde/formic acid). I'm fuelled by the synthetic challenge, not the need for drugs.
(Hive Bee)
02-06-03 20:58
No 405181
Actually before you get your panties in a bind, I was talking about the 5-MeO-AMT. If you reread my post I said "unless you are just curious", which would probably be this case. I never said it was fuelled by drugs, none of my research (as well as probably yours) is.
(Chief Bee)
02-06-03 22:34
No 405213
I haven't accused you of saying anything either, your post was not clear enough for me to sure what you meant, therefore I asked.