11-28-02 11:43
No 384271
http://www.chempensoftware.com/reactions
The reaction is a beauty:
0.2 mol of .beta.-amino isopropyl crotonate in solution in 200 c.c. of dichlorethane are added, over a period of 6 hours, to a solution, under reflux, of 0.25 mol of p-benzoquinone in 100 c.c. of dichlorethane. The water formed is progressively eliminated. After a supplementary reflux for 11/2 hours, the mixture is cooled and the 2-methyl-5-hydroxy-3-indole isopropyl carboxylate formed is filtered off.
Unfortunately the adduct or the product is 2-methyl substitued. Does anyone has any (practical) idea how this can be avoided?
"Turn on, Tune in and Drop Out"
(Hive Bee)
11-28-02 18:46
No 384340
Can the above described approach be applied to synthesise 2-Me-DET, 2-Me-DMT (#33,#33) and analogs? In that case one could treat the obtained indole with NaOH/Me2SO4, in order to convert -OH to MeO- and probably get more potent analog with different action...
"Turn on, Tune in and Drop Out"
(Chief Bee)
11-28-02 22:39
No 384377
I don't think you can work around the 2-Methyl thing, so better do like you say and use it for 2-Me-DMT etc.
How did you plan to go from the indole-3-COOH to the dialkyltryptamine?
Here is one of the refs: ../rhodium/pdf /archive/quino
(I'm Yust a Typo)
11-28-02 23:32
No 384387
But 2-Me-DMT is cool too, if you have to believe TiHKAL...
Hardcore libertarians consider the idea of a Libertarian Party dangerously socialist.
(Stranger)
11-29-02 19:02
No 384628
A professor of chemistry once told me that 2-methyl-indole's common name is "skatole," if that tells you anything.
At low concentrations, it smells like flowers, but at high concentrations, it smells like, well, shit (if you choose to believe what he said that day).
(Chief Bee)
11-29-02 19:42
No 384637
No, Skatole is 3-Methyl-Indole.
(I'm Yust a Typo)
11-29-02 20:33
No 384646
Every indole smells like shit.
Hardcore libertarians consider the idea of a Libertarian Party dangerously socialist.
(Old P2P Cook)
11-29-02 22:13
No 384658
Every indole smells like shit.
Pure indole does not "smell like shit". Certainly the "smell of shit" does contain indole as a component but so does the smell of orange blossoms. So by this reasoning I could say instead that indole smells like orange blossoms or by stretching this line of reasoning just a bit more I could say that orange blossoms smell like shit.
I know that the Merck Index says that indole has an "intense fecal odor" but the Merck Index is wrong.
Baseline Does Not Exist.
(I'm Yust a Typo)
11-30-02 00:49
No 384693
All right. 'Pure' indole has a strong, heavy rubbery/chemical smell; at least the one that I worked with. But indole degradation products (which you'll almost always get when you do some indole chemistry) surely smell shitty.
Hardcore libertarians consider the idea of a Libertarian Party dangerously socialist.
(HyperLab Bee)
12-02-02 13:48
No 385475
There was shown an ability of synthesis of indoles by reacting conc. H2SO4 with products of reaction of aniline (ArNH22) with glycidic esters.The main idea was reacting aniline with glycidic esters at high temp (in pipe bomb) on a 1st stage:
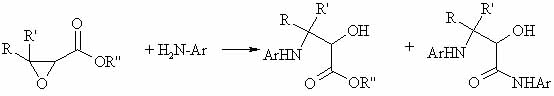
On a 2nd stage alpha-oxy-beta-anilinoester (yield 30-84%) is treated with conc. H2SO4 at high temp:
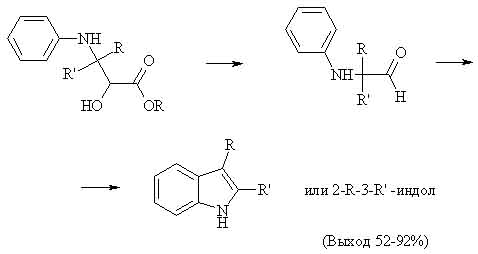
These 2 images are located at geocities,which has traffic limit,you can encounter problem opening them soon:)
However it was found out that adding conc. HCl to excess of aniline makes opening of epoxy ring more easy, thus making synth of indoles in one stage.
Experimentally reaction is very easy: excess of aromatic amine is mixed with HCl and equimolar amount of glycidic ester,then solution is heated up to 180-190 C,indoles are separated in a common way.The yield of desired products,which have alyfatic or aromatic radicals,is 68-85%.
This was presented by Narayana of HyperLab.
So having corresponding glycidic ester we could get DMT in one stage from aniline.
The main question is could anyone suggest a synth of glycidic esters,for producing exactly (unsubst.)indole???
Patents search gave nill
When I die bury me upside down so the world can kiss my ass.
(Chief Bee)
12-02-02 14:02
No 385476
Easy! Glycidic esters are formed in the base-catalyzed Darzen condensation of chloroacetic esters with aldehydes, just like in ../rhodium /2ch.dar
Barium has done some great work in this area, using aqueous NaOH with a PTC or commercial 30% sodium methoxide, UTFSE on his name for details.
(HyperLab Bee)
12-02-02 14:24
No 385483
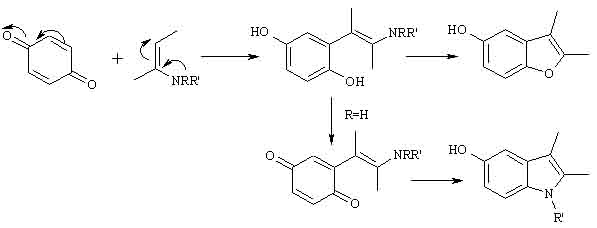
When I die bury me upside down so the world can kiss my ass.
(HyperLab Bee)
12-02-02 14:57
No 385489
You say: Glycidic esters are formed in the base-catalyzed Darzen condensation of chloroacetic esters with aldehydes...
I`ve read Barium`s thread (333551) on this topic,but missed smth., can we get them glycidic esters using this procedure without aldehyde presence,or do they cyclize only in presence of aldehyde?
When I die bury me upside down so the world can kiss my ass.