(Stranger)
12-07-02 20:34
No 387179
(Rated as: excellent)
This is a translation of the french article "Sur l'emploi d'une beta-aminoethylation dans la preparation de la tryptamine" by Robert Bucourt and Michel Vignau
Sadly this method does not work on the phenyl-magnesium bromide because it is more basic than the indole one.
Experimental
To 15.6g (0.13 mole) of methyl-magnesium bromide in ether is added 15.3g of indole (0.13 mole) in ether and then 5.6g of ethylene-imine in anhydrous xylene. The ether is eliminated completely by distillation while some xylene is added. The mixture is refluxed until all become solid. This is decomposed by addition of cold water, then some concentrated HCl is added till pH 1 and the tryptamine chlorydrate is separated. The freebase is liberated from the addition of an aqueous solution of NaOH. Yield = 9.6g (46%) mp= 116°C
Ref: Bull soc chim Fr 1961, 1190
(Chief Bee)
12-08-02 23:05
No 387563
It works for Phenylmagnesium bromide too. Search for "aziridine" and my name.
Edit: Here is the full article:
Sur l'emploi d'une
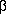 -aminoéthylation dans la préparation de la tryptamine
-aminoéthylation dans la préparation de la tryptamineR. Bucourt & M. Vignau
Bull. Soc. Chim. France 1190-91 (1961) (../rhodium/pdf /indole.aziri
(Stranger)
12-08-02 23:44
No 387581
I found yours and will fetch them in the library this week, they look interesting to say the least!
This is a translation of the rest of the french text:
To 8.15g (0.045 mole) of phenyl magnesium bromide in ether, add 2.56 g (0.045 mole) of N-methyl-ethylene-imine. After the evaporation of the ether and reflux for 2h in xylene, we hydrolyse and separate the phenylethyl-methylamine by distillation. Yield=0.2g (4%) bp 170-210°C (litt=205°C). The HCl salt melt at 152-155°C (litt=157°C)
With ethyleneimine they have got nothing at all!
So it must be protected with the bromobenzene derivative like in your ref.