05-26-03 13:11
No 435688
I was wondering if a Benzyloxy group would be cleaved in the body the same as the acetoxy groups or the Phosphorloxy to the -OH. It is a simple hydrogenase enzyme that cleaves both the acetoxy and the Phosphoryloxy correct? Why would it not also cleave the benzyloxy group to the
-OH? ANy one see What i'm getting at??
VL_
Start thinking more like a chemist and less like a criminal
(Moderator)
05-26-03 14:54
No 435706
In general enzymes are very specific for their substrates. The acetoxy, the phosphoryloxy, and the benzyloxy will most probably be cleaved by different enzymes (especially the phosphate with it's different geometry). But nobody knows exactly which specific enzymes are responsible for psilocin-ester cleavage. But I would guess that a benzyloxy would be cleaved very slowly if at all. But who knows? There might also be other (also reverse) pharmacological actions of the uncleaved compound.
Edit: I meant benzoyloxy ester, not benzyloxy ether. There is no simple metabolic way to get rid of a benzyl ether.
(Chief Bee)
05-26-03 16:33
No 435726
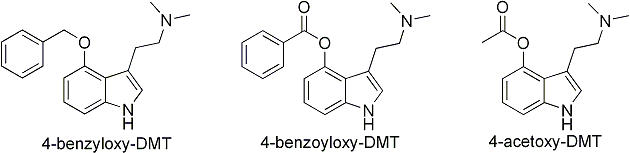
We are naturally talking about substituted 4-Hydroxy-tryptamines here. Something of great importance is that "acetoxy" and "phosphoryloxy" are the respective esters of acetic acid and phosphoric acid attached to the phenolic -OH of the tryptamine. When we have a benzyloxy group, we haven't got an ester, but an ether (the one stemming from benzyl alcohol). That is what makes it so hard to cleave compared to the others. If we had the analogous ester instead (a 4-Benzoyloxy-tryptamine), then it would much more likely cleave in the body.
Look at the structures here and compare them. The two on the left both has the Ph-CH2-O- grouping in common, but the two on the right has the same structure of their "connection" to the 4-oxygen. In this case it is the connectivity that is the most important thing, and not what kind of structure is sticking out.
(Hive Bee)
05-27-03 06:49
No 435869
According to Dr Nichols a benzyloxy group is not cleaved in vivo.
What is strange is that 4-benzyloxy-5-MeO-DMT has got serotoninergic effects on rodents....
(Chief Bee)
05-27-03 16:21
No 435948
Is it a serotonergic agonist or an antagonist? A lot of inactive tryptamines and PEAs are still binding very good to 5-HT receptors, but they function as antagonists.
(Moderator)
05-27-03 22:59
No 436016
And the other important detail is: on which receptor: 5-HT1A = bad, 5-HT2A agonist = good
(I'm Yust a Typo)
05-28-03 00:28
No 436029
What does 5-HT1a do?
(Stranger)
05-29-03 00:59
No 436271
5-HT1A receptors are expressed as autoreceptors by neurons projecting from the raphe nuclei. The monitor the synaptic 5-HT levels and modulate its release. They're also widely distributed in the cortex and amygdala and are believed to be the target for serotonergic anti-anxiety drugs.
(Moderator)
05-29-03 02:58
No 436284
5-HT1A antagonists are used as anti-anxiety medication. But as far as I know nobody knows which subjective effects a central acting and selective 5-HT1A agonists produces!