(Hive Addict)
08-04-03 12:34
No 451584
(Rated as: excellent)
The following article has been requested by Chimimanie.
E Mutschler, W Winkler. Über lokalanästhetisch und antiarrhythmisch wirksame Indol- und Indolin-Derivate. 1. Mitt. Darstellung von 1-, 2- und 3-Aminoacetyl-indolen sowie 1-Aminoacetylindolinen. Arch Pharm 311 (1978) 248-255.
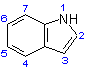
1 - 1 = -CO-CH2-R1; 2 = -R2; 3 = -R3; 7 = -R4
2 - 2 = -CO-CH2-N(C2H5)2; 3 = -CH3; 7 = -CH3
3 - 2 = -R2; 3 = -CO-CH2-R1
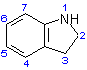
4 - 1 = -CO-CH2-R1; 2 = -R2; 3 = -R3; 7 = -R4
R1 = -N(C2H5)2, morpholine
R2 = -H, -CH3
R3 = -H, -CH3, -C2H5
R4 = -H, -CH3
Abstract - Synthesis of 1-, 2- and 3-(aminoacetyl)indoles and 1-(aminoacetyl)indolines: The chloroacetyl derivatives of various indoles and indolines, prepared by the reaction of the parent compounds with derivatives of chloroacetic acid derivatives, were reacted with amines to yield the title compounds.
EXPERIMENTAL
1-chloroacetyl-indole (5a)
5.85 g (0.05 mol) indole was dissolved in 100 mL abs. toluene. To this refluxing solution, there was slowly added drop-wise 6.4 mL (0.08 mol) chloroacetylchloride dissolved in 20 mL toluene, after which the reaction mixture was refluxed for another hour. The residue was recrystallized from ethanol after distillation of the solvent and the excess of chloroacetylchloride. Yield: 67%, mp 115°C.
1-chloroacetyl-2-methyl-indole (5b) and 3-chloroacetyl-2-methyl-indol (7b)
26.2 g (2 mol) 2-methyl-indole was dissolved in 200 mL abs toluene. A solution of 20 mL (2.5 mol) chloroacetylchloride in 50 mL abs toluene was slowly added (dropwise) to the refluxing mixture (1 h reflux). The precipitate formed during cooling down of the reaction mixture was recrystallized from toluene. Yield: 84%, mp: 224°C.
The filtrate was damped in and the residue 5b recrystallized from methanol. Yield: 8%, mp: 90°C.
1-chloroacetyl-3-methyl-indole (5c)
Prepared in analogy to 5a, employing 19.7 g (1.5 mol) skatol and 17.5 mL (2.2 mol) chloroacetylchloride. Reaction time: 10 h. Yield: 73%, mp 100°C (105°C).
1-chloroacetyl-2,3-dimethyl-indole (5d)
Prepared as described for 5a, using 14.5 g (0.1 mol) 2,3-dimethylindole and 12 mL (0.15 mol) chloroacetylchloride. Reaction time: 8 h, yield: 67%, mp 64°C.
1-chloroacetyl-3,7-dimethyl-indole (5e)
50 mL abs ether was poured over 2.4 g (0.1 mol) magnesium, and 10.9 g (0.1 mol) ethylbromide dissolved in 150 mL ether was drop-wise added. The addition rate was determined by keeping the reaction mixture at refluxing temperature. When all magnesium was used, 7.3 g (0.05 mol) 2,3-dimethyl-indole in 50 mL ether was drop-wise added and the reaction mixture brought at reflux for 2 hours when the addition was complete. The reaction mixture was cooled down and 100 mL diluted acetic acid was added while the mixture was stirred and cooled. After complete addition of the acetic acid, the reaction mixture is stirred for a further 30 minutes, after which the organic phase was isolated and washed with NaHCO3 solution. The ether layer was dried and the solvent and excess chloroacetic acid ester removed via distillation. Recrystallization of the crude end product from ethanol gave a 42% yield; mp 107°C.
3-ethyl-1-chloroacetyl-indole (5f)
Prepared as described for 5a from 7.3 g (0.05 mol) 3-ethyl-indole and 6.4 mL (0.08 mol) chloroacetylchloride. Reaction time: 3 h. Yield: 59%, mp 103°C.
2-chloroacetyl-3,7-dimethyl-indole (6)
Prepared similar as 5a, employing 4.4 g (0.03 mol) 3,7-dimethyl-indole and 8 mL (0.1 mol) chloroacetylchloride. Reaction time: 24 h. Yield: 22%, mp 175°C.
3-chloroacetyl-indole (7a)
Said compound was prepared according to Ames and Bowman (JCS (1956) 1984) from 23.4 g (0.2 mol) indole and 30 mL chloroacetylchloride. Temperature at addition of chloroacetylchloride: -35°C. Yield: 63%, mp 235°.
1-chloroacetyl-indole (8a-f)
According to the procedure published by Hach and Protiva (Chem Listy 47 (1953) 729), there was made a solution of 0.03 mol indoline and 0.04 mole chloroacetylchloride in aqueous sodium acetate. Separation of the isomeres of 1-chloroacetyl-2,3-dimethyl-indole was achieved via fractional crystallization from methanol (TLC solvent: benzene/ethylmethylketone 50+3). Isomere ratio: 70% cis, 30% trans, identified following Parnes et al (Zh Org Khim 8 (1972) 2564).
| COMPOUND | R2 | R3 | R4 | MW, formula | YIELD | MP °C |
| 8a | -H | -H | -H | C10H10ClNO (195.66) | 95% | 134 |
| 8b | -H | -H | -CH3 | C11H12ClNO (209.68) | 79% | 67 |
| 8c | -H | -CH3 | -H | C11H12ClNO (209.68) | 64% | 77 |
| 8d | -CH3 | -H | -H | C11H12ClNO (209.68) | 77% | 95 |
| 8e | -CH3 | -CH3 | -H | cis-C12H14ClNO (223.70) | 52% | 60 |
| 8f | -CH3 | -CH3 | -H | trans-C12H14ClNO (223.70) | 24% | 89 |
(1-chloroacetyl-indole corresponds to 1 - R1 = -Cl)
1-(N,N-diethylaminoacetyl)-indole (1a-f)
0.03 mol 1-chloroacetyl-indole was heated for 2-5 hours in a mixture of 0.1 mol diethylamine and 150 mL toluene. The pricipitating diethylamine.HCl, formed during the cooling down of the reaction mixture, was filtered and the filtrate treated with dilute HCl. The acidic filtrate was made alkaline with aqueous NaHCO3 and extracted with ether. After drying of the ether phase, the HCl salt of the aimed product can be obtained by introduction of HCl gas. Recrystallization from ethanol.
| COMPOUND | R2 | R3 | R4 | MW formula | YIELD | MP, °C |
| 1a | -H | -H | -H | C14H18N2O.HCl (266.77) | 44 | 190 |
| 1b | -H | -CH3 | -H | C15H20N2O.HCl (280.80) | 71 | 158 |
| 1c | -H | -C2H5 | -H | C16H22N2O.HCl (294.83) | 69 | 199 |
| 1d | -H | -CH3 | -CH3 | C16H22N2O.HCl (294.83) | 76 | 192 |
| 1e | -CH3 | -H | -H | C15H20N2O.HCl (280.80) | 63 | 176 |
| 1f | -CH3 | -CH3 | -H | C16H22N2O.HCl (294.83) | 86 | 203 |
(1-(N,N-diethylaminoacetyl)-indole, R1 = diethylamine)
1-morpholinoacetyl-indole (1g-k)
Cf. 1a-f with 0.1 mol morpholine and 0.03 mol 1-chloroacetyl-indole. (table with results skipped)
2-(N,N-diethylaminoacetyl)-3,7-dimethyl-
Said compound was prepared as described for 1a-f from 6.7 g (0.03 mol) 2-chloroacetyl-3,8-dimethyl-indole and 7.3 g (0.1 mol) diethylamine. Reaction time: 5 h. Yield: 78%, mp 186°C.
3-(N,N-diethylaminoacetyl)-indole (3a)
7.7 g (0.04 mol) 3-chloroacetyl-indole was suspended in 300 mL abs toluene and refluxed for 2 hours with 8.8 g (0.12 mol) diethylamine. The solvent is evaporated, the reaction mixture cooled down and the precipitate collected and washed with water. The residual base was recrystallized from toluene. Formation of the HCl salt was achieved by means of gaseous HCl in methanol. Yield: 74%, mp: 205-215°C.
3-(N,N-diethylaminacetyl)-2-methyl-indol
Said compound was prepared in analogy to 3a, using 6.2 g (0.03 mol) 3-chloroacetyl-2-methyl-indole and 7.3 g (0.1 mol) diethylamine. The filtrate was extracted with dilute HCl and made basic with NaOH, extracted with alot of ether and the dried ether phase freed from solvent. The base was dissolved in methanol and crystallized as the HCl salt by introduction of gaseous HCl. Recrystallization from ethanol, yield: 63%, mp 222°C.
3-morpholinoacetyl-indole (3c)
Prepared according to our description for 3a applying 7.7 g (0.04 mol) 3-chloroacetyl-indole and 10.4 g (0.12 mol) morpholine. The base was recrystallized from methanol and crystallized as the HCl salt with HCl gas. Recrystallization from aceton/ethanol. Yield: 83%, mp 210°C.
2-methyl-3-morpholinoacetyl-indole (3d)
The same procedure as for 3a but employing 8.3 g (0.04 mol) 3-chloroacetyl-2-methyl-indole and 10.4 g (0.12 mol) morpholine. Recrystallization from water/ethanol. Yield: 71%, mp 266-269°C.
1-(N,N-diethylaminoacetyl)-indoline (4a-f)
0.03 mol 1-chloroacetyl-indoline was added to a solution of 0.1 mol diethylamine in 200 mL abs toluene and refluxed for 3 to 7 hours. When the reaction mixture had cooled down, the precipitated diethylamine.HCl was collected and the filtrate extracted with dilute HCl. The acidic aqueous phase was made alkaline with sodium hydroxide and extracted with ether. The dried ether phase was treated with HCl gas and the precipitated crystals recrystallized from ethanol/water.
| COMPOUND | R2 | R3 | R4 | MW formula | YIELD | MP, °C |
| 4a | -H | -H | -H | C14H20N2O.HCl (268.79) | 93 | 162 |
| 4b | -H | -H | -CH3 | C15H22N2O.HCl (282.82) | 89 | 210 |
| 4c | -H | -CH3 | -H | C15H22N2O.HCl (282.82) | 91 | 162 |
[...]
(1-(N,N-diethylaminoacetyl)-indoline, R1 = diethylamine)
1-morpholinoacetyl-indoline (4g-i)
Same procedure as for 4a-f using 0.03 mol 1-chloroacetyl-indoline and 0.1 mol morpholine. Reaction time: 2 - 5 hours. (table containing results skipped)
Dirty old man