(Chief Bee)
09-27-03 17:47
No 461398
(Rated as: good read)
For example, 2-dimethylaminoethanal would give DMT, and alpha-bromo-acetaldehyde would give 3-(2-bromoethyl)-indole, which in turn would be ready for alkylation by any dialkylamine to give the corresponding N,N-dialkyltryptamine.
A general method for C3 reductive alkylation of indoles
Anu Mahadevan,Howard Sarda, Mario Gonzalez, John C. McKew
Tetrahedron Letters 44(24), 4589-4591 (2003) (../rhodium/pdf /reductive.3-
DOI:10.1016/S0040-4039(03)01010-4
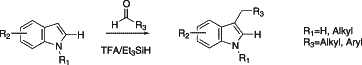
Abstract
General indole C3 reductive alkylation conditions have been developed. The scope of this reaction includes C2 unsubstituted indoles, aryl and alkyl aldehydes, as well as N–H and N-alkyl indole substrates.
(Chief Bee)
09-28-03 02:47
No 461398
(Rated as: good read)
For example, 2-dimethylaminoethanal would give DMT, and alpha-bromo-acetaldehyde would give 3-(2-bromoethyl)-indole, which in turn would be ready for alkylation by any dialkylamine to give the corresponding N,N-dialkyltryptamine.
A general method for C3 reductive alkylation of indoles
Anu Mahadevan,Howard Sarda, Mario Gonzalez, John C. McKew
Tetrahedron Letters 44(24), 4589-4591 (2003) (../rhodium/pdf /reductive.3-
DOI:10.1016/S0040-4039(03)01010-4
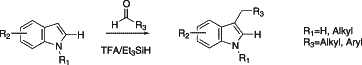
Abstract
General indole C3 reductive alkylation conditions have been developed. The scope of this reaction includes C2 unsubstituted indoles, aryl and alkyl aldehydes, as well as N–H and N-alkyl indole substrates.
(Chief Bee)
10-15-04 03:11
No 535894
(Rated as: good idea)
Their examples does not include nitro-alkenes, but all alkenes with any electron-withdrawing group whatsoever worked in their synthesis, so it is highly likely that this can be used to produce 1-(3-indolyl)-2-nitropropenes in a single step from indoles and 3-acetoxy-2-nitro-propene. This can in turn is made by condensing formaldehyde with nitromethane (yielding 2-nitro-propane-1,3-diol), acetylating the diol with Ac2O or AcCl and distilling in vacuo to give the nitroalkene (se refs at the bottom).
Palladium-catalyzed functionalization of indoles with 2-acetoxymethyl substituted electron-deficient alkenes
Shengming Ma, and Shichao Yu, Tet. Lett. 45(45), 8419-8422 (2004), DOI:10.1016/j.tetlet.2004.08.178

Abstract
A new functionalization of indoles via palladium-catalyzed reaction of indoles and 2-acetoxymethyl substituted electron-deficient alkenes is reported. The reaction was carried out under neutral condition and no isomerization of the carbon–carbon double bond was observed.
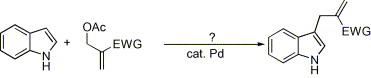
Preparation of 3-acetoxy-2-nitro-propene:
Nitromethane + Formaldehyde --1:NaOH--2:Acetylation--> 3-acetoxy-2-nitro-propene
M.B. Frankel, Tetrahedron Suppl. 4, 213-217 (1963)
2-nitro-propane-1,3-diol + acetyl chloride --CH2Cl2--> 1,3-diacetoxy-2-nitro-propane --180°C/70-100mmHg--> 3-acetoxy-2-nitro-propene
Klager,K.; Monatsh. Chem. 96, 1-8 (1965)
2-nitro-propane-1,3-diol + acetic acid anhydride -> 1,3-diacetoxy-2-nitro-propane
Carbohydr. Res. 310(3) 191-202 (1998)
The Hive - Clandestine Chemists Without Borders