(Hive Bee)
04-30-04 12:08
No 504026
(Rated as: excellent)

Tetrahedron, 2004, 60, 4567-4578
DOI:10.1016/j.tet.2004.03.073
Michael Decker, Thi Thanh Huyen Nguyen and Jochen Lehmann
Abstract
The mechanism of lactamization of corresponding lactones was investigated by means of gas chromatography and synthesis of possible intermediates as references. Lactones react with amines via the amino acid with subsequent elimination of water to the corresponding lactams. In the first step, also hydroxyamides are in equilibrium with the lactones and amines, respectively, which are not able to form the amide though. This mechanism opens a new approach for the synthesis of N b-disubstituted tryptamines.
Keywords
Lactamization; Lactones; Amino acids; Hydroxyamides; Tryptamines; Hallocinogens.
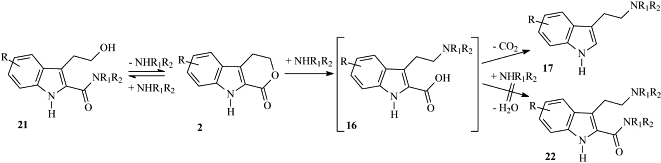
Scheme 6. Formation of N[beta]-disubstituted tryptamines (17) from 4,9-dihydropyrano[3,4-b]indol-1(3H)-ones
Several new tryptamines were synthesized, including some which are able to displace the 5-HT2 antagonist ketanserin from the receptor. According to the pharmacological data these substances might bee potential hallucinogens but from the data it is not clear wether these compounds are agonists or antagonists on the 5-HT2a receptor.
The authors also present a novel route to DET.
The tendency is to push it as far as you can
(Chief Bee)
04-30-04 13:45
No 504044
That's interesting. Now we only need to see how hard it is to synthesize the tricyclic starting material 2:
The 4,9-dihydropyrano[3,4-b]indol-1(3H)-ones
13. Lehmann, et. al, Arch. Pharm. Pharm. Med. Chem. 1987, 320, 1202–1209.
14. Lehmann, et. al, Arch. Pharm. Pharm. Med. Chem. 1987, 320, 698–704.
The Hive - Clandestine Chemists Without Borders
(acetaminophanatic)
04-30-04 18:54
No 504087
gentle Friedel-crafts alkylation of the hydrolyzed product of a Reissert synthesis with ethylene chlorohydrin, followed by dehydration.
i.e 2-nitrotoluene --[reissert synthesis]-->
ethyl indole-2-methanoate --[F.C. Alkylation]-->
ethyl 3-hydroxyethyl indole-2-methanoate --[gentle hydrolysis or heating??]-->
cyclized lactone intermediate.
Then lactamize with the alkylamine of choice and decarboxylate. Nice!
Molecule: alkylate ("OC(=O)c1nc2ccccc2c1.ClCCOH>>OC(=
Molecule: dehydrate ("OC(=O)c1nc2ccccc2c1CCO>>O3C(=O)c
Mmm, mmm good.
Then again, would the presence of that methanoic acid group screw up the alkylation procedure?
I've been chased by both cops and robbers. So what does that make me?
(Chief Bee)
04-30-04 19:40
No 504096
Then again, would the presence of that methanoic acid group screw up the alkylation procedure?
Catastrophically. Carboxylic acids are more active than alkyl chlorides in FC a(c/lk)ylations.
The Hive - Clandestine Chemists Without Borders
(acetaminophanatic)
04-30-04 21:50
No 504114
then what?
EDIT:
Seems like the presence of an acid ester in the 2-position isn't going to change the alkylation pattern, at least how I draw the resonance structures. That's good.
EDIT2:
Post 268709 (flipper: "reissert indole synt", Tryptamine Chemistry) mentions http://www.orgsyn.org/orgsyn/prep.asp?pr
The acid or its ester serves as a readily accessible indole capable of electrophilic substitution at the 3-position
(H. Fischer and K. Pistor, Ber., 56B, 2213 (1923), R. H. Cornforth and R. Robinson, J. Chem. Soc., 680 (1942))
So there you have it.
I've been chased by both cops and robbers. So what does that make me?