(Hive Addict)
05-10-04 21:17
No 506265
(Rated as: excellent)
A versatile synthetic methodology for the synthesis of tryptophols
Simon J. Garden, , Rosângela B. da Silva and Angelo C. Pinto
Tetrahedron, 58(42), 8399-8412 (2002) (../rhodium/pdf /isatin2trypt
DOI:10.1016/S0040-4020(02)01048-7
Abstract
Tryptophols have been obtained in high yields by the reduction of 3-substituted-dioxindoles (obtained by the aldol condensation reaction of ketones with isatins or by a modified Knovenagel malonate condensation) using a borane tetrahydrofuran complex. The reported methodology offers distinct advantages over existing methods for the synthesis of these compounds, including consistently greater yields, diastereoselective syntheses and the possibility for the synthesis of a wide range of structurally different tryptophols. The reduction reaction was found to proceed via an intermediate 1,3-diol-oxindole, which was obtained diastereoselectively and, which was subsequently reduced to the corresponding tryptophol.
Graphical Abstract
A diverse range of tryptophols can be readily prepared by the BH3·THF reduction of dioxindole derivatives. Intermediate diols are produced by diastereoselective reduction reaction.
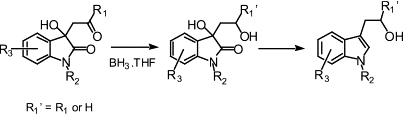
Author Keywords: isatin; reduction; borane tetrahydrofuran complex; diastereoselective reduction; tryptophol
http://www.geocities.com/eric_vornoff/go
(Hive Bee)
05-12-04 22:13
No 506708
Okee nice info but I would rather stick to this one.
I do not like procedures which start with Indoles.
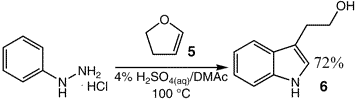
(Chief Bee)
05-12-04 22:24
No 506714
I do not like procedures which start with Indoles.
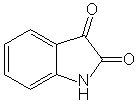
Where did you get that from? The procedure posted above starts with isatin, which is a lot cheaper than indole.
The Hive - Clandestine Chemists Without Borders
(Hive Addict)
05-13-04 20:14
No 506908
as it is readily made by oxidation of indigo with nitric acid or a mixture of nitric and chromic acids.
Check out these golden oldies:
O. L. Erdmann:
J. Prakt. Chem (1) 19, 321 (1840)
J. Prakt. Chem. (1) 22, 257 (1841)
J. Prakt. Chem. (1) 24, 1 (1841)
A. Laurent:
Ann. chim. phys. (3) 3, 372 and 469 (1840)
Rev. sci. ind., september 1842
J. Prakt. Chem. (1) 25, 434 (1842)
There are quite some interesting synthetic routes to (substituted) isatin(s) too.
For a good review of the chemistry of isatin, see Chemical Reviews 34/35 (1944) p 393-434
On a side-note: When I first smelled isatin, I found it to have the exact smell of the watercolor paint I had as a kid. Could isatin itself be used as a pigment too (as in red watercolor paint
http://www.geocities.com/eric_vornoff/go
(Chief Bee)
05-13-04 21:36
No 506926
When I first smelled isatin, I found it to have the exact smell of the watercolor paint I had as a kid.
Really? That was the exact impression I got when I smelled phenol for the first time. How would you compare the smell of isatin with phenol?
The Hive - Clandestine Chemists Without Borders
(Hive Addict)
05-13-04 22:24
No 506941
..as accurrately as possible, but I would say isatin has more of a "groundish" or "soil-like" undertone.
This always comes to mind:

Considering that many indigo and isatin derivates are used as pigments, that was what made me think of that possibility. However, I haven't found any such use for isatin yet, I'm sure it would make a nice red color though..
http://www.geocities.com/eric_vornoff/go
(Hive Bee)
05-16-04 13:01
No 507462
I have been reading and came to the conclusion that I liked it.
Good find.
90% yield is fucking great.
(Hive Bee)
05-16-04 19:15
No 507518
How much does that Borane costs??
I dont like expensive methods.
(Chief Bee)
05-16-04 20:32
No 507529
Why don't you check with the suppliers you are able to buy from? Prices vary a lot.
A cheap method for the production of BH3·THF is to react NaBH4 with BF3·Et2O in THF, see verious Varma & Kabalka papers here and/or on my page for the exact procedure.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
05-16-04 21:33
No 507544
1 liter 1M solution BH3.THF in THF costs about 250 Dollar.
Well a 1 mole batch needs 4 liters of that stuff. Costs 1000 dollars for the BH3.THF solution alone.
A 1 kilo batch needs about [I guess] 30 liters of the stuff. That's 7500 Dollars.
It is atleast cheaper then when you make Tryptophol from PhenylHydrazine. That stuff costs 1300 Dollars every 100 grams.
But it's still a pain in the wallet.
I plead for a Economics forum on this site.
(Hive Bee)
05-16-04 22:23
No 507545
Prices of chemicals don't scale linear with amount. Also, if you seriously think of making 1 kg batches, you'll also need dedicated equipment. Think 30l rotovapors and such. The people who sold you that stuff usually also know were you can get chemicals cheaply :-)
Besides, scaling up a reaction from mmol to mol-scale often comes with quite unexpected problems ('how can I handle excess heat/gas evolution/byproducts').
(Moderator)
05-16-04 23:25
No 507549
Make it yourself for 1/20 of that price. UTFSE. Those expensive chemicals and formulations are for lazy chemists in rich companys or institutions who don't care about the price.
(Heavyweight Chempion(eer))
05-17-04 00:03
No 507555
Borane is easily made in situ from sodium borohydride and iodine or 100% sulfuric acid. There is something posted here using such a method to reduce amino acids with in situ made borane from iodine and sodium borohydride in THF, I think Rhodium posted that one. UTFSE.
Severe Aztecoholic and President of Sooty's fanclub - Sooty for President!!