(Chief Bee)
05-21-04 19:09
No 508709
(Rated as: excellent)
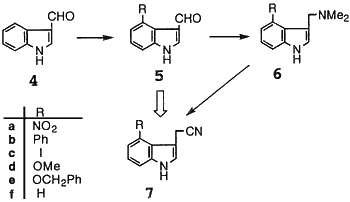
Simple One Step Syntheses of Indole-3-Acetonitriles From Indole-3-Carboxaldehydes
Fumio Yamada, Tomoko Hashizume, and Masanori Somei
Heterocycles 47(1), 509-516 (1998) (../rhodium /indole
Abstract
One step conversion method of indole-3-carboxaldehydes into indole-3-acetonitriles is developed. Applying the method, 4-nitro- (7a), 4-phenyl- (7b), 4-iodo- (7c), 4-methoxy- (7d), and 4-benzyloxyindole-3-acetonitrile (7e) are available in two steps from indole-3-carboxaldehyde (4).
Edit: Is there anyone out there who would make an educated guess regarding the reaction mechanism, and if this procedure would work for benzaldehydes too, and not just infole-3-aldehydes?
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
05-22-04 19:11
No 508905
Is there anyone out there who would make an educated guess regarding the reaction mechanism, and if this procedure would work for benzaldehydes too, and not just infole-3-aldehydes?
They said in the preambule: In order to accumulate basic knowledge, we chose 4-nitroindole-3-carboxaldehyde9 (5a) as a substrate and tested various trials employing cyanating reagents in the presence of reducing agents, such as Me3SiCl-NaI-KCN-Et3SiH, Me3SiCl-NaI-KCN-NaBH4, Me3SiCN-NaBH4, and so on. During these studies,8 we observed that simple treatment of 5a sequentially with NaBH4, and then with NaCN in MeOH, produced 4-nitroindole-3-acetonitrile.
Here, KCN reacts with the Ar-CHO forming the alpha-hydroxy cyanide, which is consequently transformed to the Ar-CHOSi(CH3)3-CN & the NaBH4 hydride reduction generates the Ar-CH2-CN.
Procedure: NaBH4 (1.3 mol eq.) was added to a solution of indole-3-carboxaldehyde in MeOH and NH2CHO. After stirring at rt for 1 h, NaCN (10 mol eq.) was added to the reaction mixture and the whole was refluxed on oil bath at 100°C for 5 h with stirring
In this procedure, they wait 1 hour before adding the NaCN, so it is reasonable to expect that further reaction occurs on the Ar-CH2OH. I speculate that the alcohol reacts with the formed B(OMe)3 (from NaBH4 & MeOH) to yield Ar-CH-OB(OMe)2 and that nucleophilic displacement with cyanide or NH2CHO generates 7a and 9a respectively. I guess the rather large excess of cyanide is used to favor the formation of 7a (instead of the solvolysis --> 9a).
Now, the question that remains is: how is 8a formed?
If this would be the rationale after the reaction, I don't see why Ar should be restricted to indole.
Comments on this proposed mechanism are appreciated
By the way: this is a rather nice & ingenious procedure