03-07-02 18:01
No 278429
I was looking in the library for info on the subject, when i stumbled upon these:
CA 95:96939 The urushibara Ni catalyst was improved in activity by increasing the amount of Zn, and the amount of HCl. Also increased activity by upping the cementation temperature.
They report a decreased activity when the amount of Al and AcOH is increased.
The original article is in Russian, i believe
Also, CA 79:108478 says that refluxing the ppt. Ni in alcohols gives about the same activity as acid-activated Ni
It's maybe not much but i put it here for the sake of the search engine.
e109
(Official Hive Translator)
03-08-02 05:56
No 278746
Element 109 - could you, please, possibly-maybee scan and upload that article in Russian? I'm very interested in this topic and will gladly translate it , if you will.
Yours,
Antoncho
(Master Searcher)
03-09-02 17:44
No 279529
(Rated as: excellent)
Here's CA vol. 79, 1973 108478g
108478g New type of Urshibara Nickel catalyst (U-Ni-N). Activation of the precipitated nickel (precursor of Urushibara nickel catalysts) by refluxing with alcohols. Kajitani, Masatsugu; Okada, Junko; Ueda, Tetsuya; Sugimore, Akira; Urushibara, Yoshiyuki (Fac. Sci. Technol., Sophia Univ., Tokyo, Japan). Chem. Lett. 1973, (8), 777-80 (Eng). A new method is described for activating pptd. Ni by refluxing with alcs. The pptd. Ni activated in this manner exhibits a catalytic activity corresponding to acid-activated pptd. Ni. The catalyst is useful for the selective hydrogenation of 1-octene (low isomerization) and cycloolefins.
And CA Vol. 95, 1981 95:96939y and 95:96940s
95:96939y Optimization of Urushibara nickel catalysts for hydrogenation. Part I. Effect of preparation conditions on the spectific activity of the catalyst. Maksimova, N. A. ; Fasman, A. B. (Inst. Org. Katal. Elektrokhim., Alma-Acta, USSR). Izv. Akad. Nauk Kaz. SSR, Ser. Khim. 1981, (3), 26-31 (Russ). A math. model was derived to describe the activity of Urushibara Ni catalysts in hydrogenation of HC÷CCMe2OH as a function of the prepn. conditions. Improved catalysts were obtained by increasing the Zn content, the concn. of the HCl used to treat the Zn before the onset of cementation, and the cementation temp., and by decreasing the Al content and AcOH concn.
95:96940s Optimization of Urushibara-type nickel catalysts for hydrogenation. Part II. Effects of preparation conditions on the selectivity of hydrogenation of dimethylethynylcarbinol. Fasman, A. B.; Maksimova, N. A.; Pel'menshtein, B. Ya. (Inst. Org. Katal. Elektrokhim., Alma-Acta, USSR). Izv. Akad. Nauk Kaz. SSR, Ser. Khim. 1981, (3), 31-4 (Russ).
A simplified math. model was derived to describe the effects of the conditions of the prepn. of Urushibara Ni catalysts on the selectivity of HC÷CCMe2OH hydrogenation with them. The max. selectivity for CH2CHCMe2OH was 93% , obtained using 20% Pd2+, 40% Mn2+ and 1:10
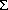 (Ni, Pd, Mn)/Al at pH 6.5, cementation at 40º for 30 min, leaching at 40º with 20% NaOH for 20 min, Al particle size 0.2-0.4 mm, and wash-water temp. 20º.
(Ni, Pd, Mn)/Al at pH 6.5, cementation at 40º for 30 min, leaching at 40º with 20% NaOH for 20 min, Al particle size 0.2-0.4 mm, and wash-water temp. 20º.Note: ÷ means triple bond here.
Where did I get the
 from?
from?http://www.geocities.com/dritte123/PSPF.
(Hive Bee)
03-10-02 20:44
No 280178
Antoncho: I'm sorry I don't have access to any of the full articles , my library only stores J.org.chem.USSR no other russian nor japanese papers. Interlibrary loan is an option but right now i don't have the $$ required
e109