04-25-02 07:46
No 301255
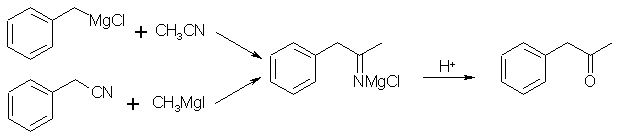
After Hydrolysis of the grignard reagent eg with dilute HCl What are the side products? Im currious to understand what happends to the NMgCl complex.
Can a bee be visa vis its entity when half a bee philosophically must ipso facto half not be?
(Chief Bee)
04-25-02 10:26
No 301301
The =NMgCl complex reacts with HCl to give MgCl2 and the imine =NH, and the imine is then hydrolyzed by H2O/HCl to give the ketone =O and NH4Cl.
(Hive Bee)
04-25-02 17:23
No 301411
Whow thats great Rhodium, its easy to see how water is detramental to NaBH3 reductions of P2Ps.
Can you explain the attraction/reaction of the nitrogen in the methyl cyanide to the grignard set (MgCl)?
To any bees interested in grignard reactions http://www.chem.ucalgary.ca/courses/351/
Can a bee be visa vis its entity when half a bee philosophically must ipso facto half not be?
(Chief Bee)
04-26-02 04:12
No 301603
One of the resonance forms of a nitrile is R-C+=N- and the grignard reagent is equivalent to a R- anion, therefore it adds to the nitrile carbon.