03-19-04 11:16
No 496109
That have the same salt?
Are all amines alkoloids?
Thanks much
ITP
P.s Im sorry for asking the same basic question in 3 forums, but i think they are different enough to warrent it.
(Stranger)
03-19-04 14:04
No 496122
Make the freebase from it and distill, as they probably have different boiling points.
All amines are not alkaloids.
(Hive Addict)
03-19-04 22:57
No 496190
the term alkaloid is used for amines found in plants that have strong physiological effects.
Which 2 amines are they? You could distill, but maybe, depending on which ones they are, you could use some other method.
3
(Stranger)
03-19-04 23:11
No 496194
Im trying to find a way to seperate DXM HBr and Pseudo in a syrup. There are two ways I see you could do it with these particular amines. First wash the hell out of the syrup with a np, then, before an A/B reaction raise the ph gradually untill the first pKa is met and one of the salts crash out. Then filter, and raise the ph till the other salt crashes out and filter again. I know the pKa for PseudoHCl is 9.22, I dont know DXM HBrs.
Or, do the A/B extraction. While they are still in fb, slowly lower the ph untill the first fb turns into a salt, drain that aqueous layer, and repeat for the other fb.
The boiling points of both amines are close enough that you cant distill them out.
(Hive Addict)
03-19-04 23:31
No 496198
The pKa is the point at which 50% of the amine is ionized & not. The binding has also to do with the acid used, and you might want to look at solubility charts, what solvents will dissolve which.
3
(Hive Bee)
03-22-04 02:34
No 496531
ion exchange resin. Its very cheap, more than likely not watched and completey reusable. Load onto Dowex or Amberlite etc then elute with differing strengths of ammonia solution.
Fan of Shulgin
Peace, love and empathy
(HyperLab Bee)
03-22-04 11:28
No 496607
First, add NaOH and extract DXM+pseudo from your syrup into petroleum ether.
Second, reextract DXM+pseudo to aq. HBr
Third, evaporate you HBr solution till crystals just starts forming. Cool your solution to 0 deg. Filter off the DXM-HBr. Pseudo and some DXM there is in a solution.
It is because DXM-HBr solubility in cold water is about 1.5g/100 ml, but pseudo solubility is much more.
Several recrystallizations will produce pure products.
With best regards,
Dennis Prochko aka Wolf
(Stoni's sexual toy)
03-30-04 14:28
No 498090
Chromatography.
BUSH/CHENEY 2004! After all, it ain't my country!
(Synaptic Self-Mutilator)
03-30-04 19:04
No 498123
Os, I wanted to say A/B then chromatography so badly as well. The problem is you need:
1) shortwave uv lamp for detection or iodine ../rhodium /equipme
2) tlc plates
3) silica
4) column
5) pressurized air
6) good solvent system
That's all relatively tough for a clandestine chemistry to put together in their lab.
(Stoni's sexual toy)
03-30-04 19:19
No 498124
> 1) shortwave uv lamp for detection or iodine
or H2SO4, or countless other easily available detection reagents...
> 2) tlc plates
Make them yourself from glass slides and absorbent if they are that hard to get.
> 3) silica
or other absorbents like Al2O3, chalk, paper...
> 4) column
Filtration funnel will do. A small microscale chromatography column can also be improvised by using a pasteur pipette. Or a glass or plastic tube. Same is true for bigger columns.
> 5) pressurized air
What for? You don't need that.
> 6) good solvent system
Hardware store plus a little experimentation. It's really not that hard to figure one out that works.
BUSH/CHENEY 2004! After all, it ain't my country!
(Stranger)
03-30-04 21:05
No 498131
@jsorex
Actualy, alkaloids ('real alkaloids') are not the same as amines.
They are heterocyclic nitrogen containing molecules, syntesized from amino acids.
Proto-alkaloids are amines (non-heterocyclical) derived from amino acids.
See this picture:
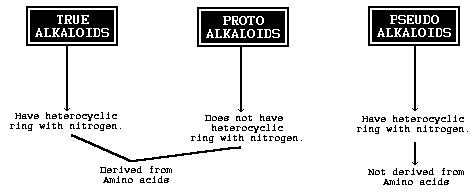
Most substances discuesed here are proto-alkaloids.
Not all alkaloids are physiological/psygological active (nucleic acids are alkaloids too), only the most interesting ones
Further alkaloids are not only found in plants: they are also found in insects and some micro-organisms.
Tough to the best of my knowledge noone made drugs from insects or micro-organisms.
None are more hopelessly enslaved than those who falsely believe they are free.
(acetaminophanatic)
04-12-04 05:45
No 500297
Os, somehow I don't think paper and H2SO4 are going to get along very well...nor the chalk...
I very much would like to do some chromatography myself, but found a sort of difficult catch:
If I use TLC plates, H2SO4 (cheap & easy) will work. But where to get the plates?
If I use paper (got lots of good filter paper for that), then what to use as the detection agent? Iodine?
Catching a buzz @ the Hive
(Stoni's sexual toy)
04-12-04 17:40
No 500364
> If I use TLC plates, H2SO4 (cheap & easy) will work.
> But where to get the plates?
Now that's really hard question. Maybe you could try to buy them?
or you make your own by applying some SiO2 on microscopy glass slides, or on some plastic or aluminum foil?
> If I use paper (got lots of good filter paper for that), then what
> to use as the detection agent? Iodine?
I guess. Try it out.
BUSH/CHENEY 2004! After all, it ain't my country!
(Hive Addict)
04-12-04 19:53
No 500377
fawaka ,
I know all of this, I took the definition out of a general pharmacognosy book. I thought that to be an easy explanation for this case. Also the book says that all alkaloids have physiological effects otherwise they are just amines.

(Synaptic Self-Mutilator)
04-12-04 22:09
No 500393
Okay, there are a lot of solvent systems to choose from... and a lot of adsorbants.... silica gel, talc, fine ground activated charcoal, diatomaceous earth (celite), molecular sieves, aluminum oxide, magnesium oxide, magnesium sulfate, sodium bicarbonate, starch, sucrose. But you must select your adsorbant based on what you're separating. And yeah, there's a ton of solvent systems you could use... hexane (or other light hydrocarbons), toluene, dcm, ethyl acetate, ethanol, methanol, acrylonitrite, water... (basically any two solvents, one more polar, one less polar...) but that depends on your adsorbant and on what you're trying to separate.
But I still say performing column chromatography is nearly beyond most clandestine bees... unless of course, they've got real TLC plates (not homemade ones), good adsorbants, quality solvents and a reliable detection method (preferably UV light or iodine). You've also got to have a rack of test tubes, an appropriately-sized column...
Have you ever seen how slowly a solvent will drip thru a column without either using suction or pressure? It's rather slow. I wouldn't think it possible, but I'll take your word for it.
Your view is interesting Osmium, I just happen to disagree with you.
(Stoni's sexual toy)
04-13-04 10:41
No 500535
> I still say performing column chromatography is nearly beyond
> most clandestine bees...
No it's not. I don't understand why most of you are afraid of chromatography. This procedure is extremely simple. When I was a kid I got a chemistry set, and it contained a simple burette which was also used as a chromatography column.
> unless of course, they've got real TLC plates (not homemade ones),
For years scientists have prepared their own plates. Today they can be ordered from every lab shop. It's not even a suspicious item.
> good adsorbants, quality solvents and a reliable detection method (preferably
> UV light or iodine). You've also got to have a rack of test tubes, an
> appropriately-sized column...
All you need is a buchner funnel. No need for a real huge column. But even they can be made at home, it's only a glass pipe. For microscale separations all you need is a friggin 10c pasteur pipette!
And test tubes... where I come from we don't even clean them, they are use once and discard items, just like pipettes. Just buy a box with 500
BUSH/CHENEY 2004! After all, it ain't my country!
(Synaptic Self-Mutilator)
04-13-04 20:38
No 500614
Ok, I want to prove you right Osmium. I really do. I'd love to setup a simple flash column chromatography setup. I'd love to separate the various colors of dyes from ink in a marker.
Solvent? 70% IPA. (other suggestions?)
Adsorbant? 5cm of celite. (other suggestions?)
Plug? Cotton batting
I'll use an addition funnel to run it as I don't have a column at home... do you think it'll work? Without suction or pressure to force the liquid through?
This sort of experiment might also be a good practice run for those other bees also intimidated to run chromatography in their labs. (plus it requires no UV detection as the dyes are readily visible)
(Hive Bee)
04-13-04 22:16
No 500641
Although i've never deliberately attempted to make a chromatograph I probably should, it hasn't escaped my attention how, for example, there was distinct colour bands in a syrian rue filtration on the filter paper, first a red area and further out this fluorescent yellowish colour...
I keep trying acid/base extractions on unknown plant materials, not that i've tried *that* many times, but it would probably make a lot of sense for me to get some idea of how many different things are in it (and maybe work out why i don't seem to be getting decent results) and do some experiments with packing, mobilisers and eluents and extraction methods and variations to find the best way to get good info. Silicotungstic acid probably would be a faster way for me to find out... but still. I can imagine mixing some kind of colour indicator in, I2 or whatever is suitable to get the alkaloid band to become visible.
One nice thing about this country i'm living in is that syrian rue is seemingly still legal here, and because of the high content of highly coloured materials in it (even a UV active one), it would be a good place to start learning about packing columns evenly and all that, doing simple paper chromatographs and making glass plates. Plus i could diagnose variations in method as to how much purity i'm getting.
I can really see how TLC and microscale chromatography really are indispensible tools for figuring out what you've got mixed into a solution or whatever, to see how pure you've got something, or how far your reaction is progressing.