07-09-04 01:13
No 518259
Perhaps I shouldnt be posting another question so soon after that last one but I cant really help myself:
If mescaline is synthesized from tyrosine, how do the hydroxys (ultimately methoxys) get into the 3 and 5 positions? Why are there not side reactions producing 2,4,6 trimethoxy PEA and 2,4,5 trimethoxy PEA? It would seem like that would happen considering that alkyl, methoxy, and hydroxy groups are all ortho, para directing. Why is it that we humans have to start from an aldehyde to get what we want in the meta position, but mother nature doesnt have to? Are there specialized protiens that allow for tighter control of substituant directing than can be gotten in a conventional lab?
For that matter, how does tyrosine hydroxylase make dopa without a side product of 2,4, and 4,6 dihydroxy PEA?
Could bees study these principals of how the protiens catayze so selectively and put them to their own use?
(Hive Adickt)
07-09-04 07:10
No 518334
Thats the beatu of enzymes. But for us humble humans, who don't have the time to construct a 500 aminoacid cave to make chemestry in, we have to doo it the hard way.
(Hive Addict)
07-09-04 08:45
No 518340
Next time you go to the library, search for books discussing the shikimic acid pathway and phenylpropanoid biosynthesis. But it will be a very hard topic if you don't have sufficient knowledge on both biology and biochemistry. But interesting nonetheless.
For a while, I was interested to know how 2.4.5-trimethoxyphenylpropanoids were biosynthesized, but I didn't find much information (though it might have been I didn't look good enough). There are zillions of articles discussing phenylpropanoid biosynthesis with substitutions in the 2-, 4-, 3.4- and 3.4.5-positions. It seems to be something every plant is capable of doing by default (i.e. without the Divine intervention of God, which changed some genes and made several plants synthesize "odd molecules").
Dangerous terrorist placing sperm mines in Anglo-saxon pussy
(Hive Bee)
07-09-04 10:43
No 518359
Next time you go to the library, search for books discussing the shikimic acid pathway and phenylpropanoid biosynthesis. But it will be a very hard topic if you don't have sufficient knowledge on both biology and biochemistry. But interesting nonetheless.
Will do. Actually I am way better at biology than chemistry, so it might be worth a shot. I never heard of shikimic acid, but it does seem to have a very interesting structure. A quick google search found this interesting link: http://www.friedli.com/herbs/phytochem/s
In the reaction on the right, I wonder if it is the same enzyme that that removes the meta-hydroxy groups as adds the COCOOH. If not that would be ![]() .
.
I wonder how many of these genes have been fully sequence and the appropriate primers for splicing into yeast found.
Surely its not as simple as inserting the gene, getting it expressed, and adding the appropriate precusor the the transgenic yeast, is it? Ohhh the things that could be done.... Indole-ethylamine n-methyltransferase here we come! (that has been expressed in yeast). Im sure the bees here have read these references, but just in case:
Michael A. Thompson Eunpyo Moon, Ung-Jin Kim, Jingping Xu, Michael J. Siciliano,
and Richard M. Weinshilboum, Human Indolethylamine N-Methyltransferase: cDNA Cloning and
Expression, Gene Cloning, and Chromosomal Localization, Genomics 61, 285–297 (1999)
and
Michael A. Thompson and Richard M. Weinshilboum, Rabbit Lung Indolethylamine N-Methyltransferase
cDNA AND GENE CLONING AND CHARACTERIZATION, THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 273, No. 51, Issue of December 18, pp. 34502–34510, 1998
(Newbee)
07-09-04 14:24
No 518384
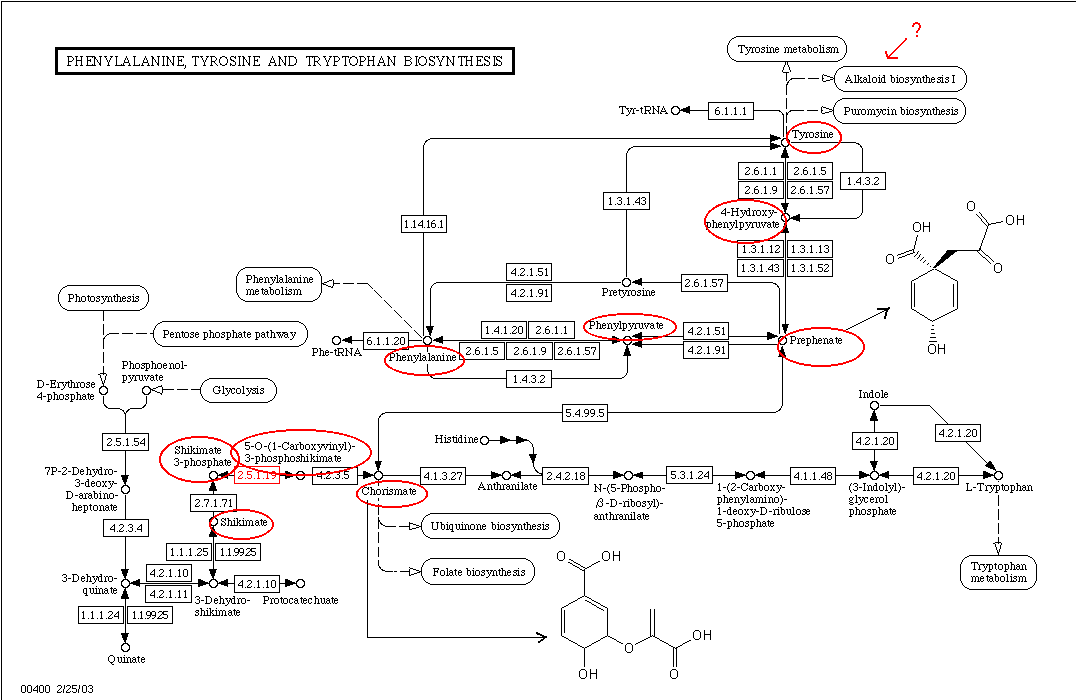
Brr, not so simple. Shikimic acid loses its 3- and 5-hydroxy groups very early. Yes, psycosmo, it`s not the same enzyme but you can see that enzymatic shikimic -> 3,4,5-trihydroxyphenylpyruvic appears to be imposible (or at least very hard
I remembered epinephrine biosynthesis: tyrosine -> 3,4-dihydroxyphenylalanine -> dopamine -> norepinephrine -> adrenaline. Maybe mescaline will find its place somewhere here.
The chart is from http://www.genome.ad.jp.
(Hive Bee)
07-10-04 02:26
No 518464
I remembered epinephrine biosynthesis: tyrosine -> 3,4-dihydroxyphenylalanine -> dopamine -> norepinephrine -> adrenaline. Maybe mescaline will find its place somewhere here.
From this link: http://www.usm.maine.edu/~newton/Chy251_
It would seem that mescaline biosynth diverges from the more familar pathway you cite early on. The above link says that for mescaline, tyrosine is decarboxylated, then dopamine is produced by 3-hydroxylation (as opposed to dopa decarboxylase), then dopamine is methylated (presumably by Catechol-O-methyltransferase), and THEN the 5-hydroxylation occurs. So whatever enzyme does this is the one to be interested in.
It just seems so paradoxical from what little I know about chemistry because the 3-methoxy is is pushing the reaction to the 2-position via ortho-direction and the 6-position via para subsitution, and the alkylamino group is also pushing the reaction towards 2,6 subsitution. The 4-hydroxy is the only thing to push the reaction in favor the the 5-position. I could see steric hinderence preventing 2-hydroxylation, but not 6-hydroxylation.
So what enzyme is this, that can 5-hydroxylate 3-o-methyldopamine? If it has not yet been sequenced perhaps looking for genes homologous with tyrosine hydroxylase would be a good place to start?
And thanks for the cool diagram and link, thats one complicated set of pathways ![]() . How is it that reactions in nature can be at the same time so incredibly Rube Goldberg, but at the same time so delightfully elegant?
. How is it that reactions in nature can be at the same time so incredibly Rube Goldberg, but at the same time so delightfully elegant?
And if I might ask one other question, why are 3,5 hydroxys so vulnerable to removal? Is this a general rule, or only when there is a 1-carbonyl group? What conditions favor/disfavor 3,5 dehydroxylation?
(Newbee)
07-10-04 11:37
No 518521
Some more drawings...
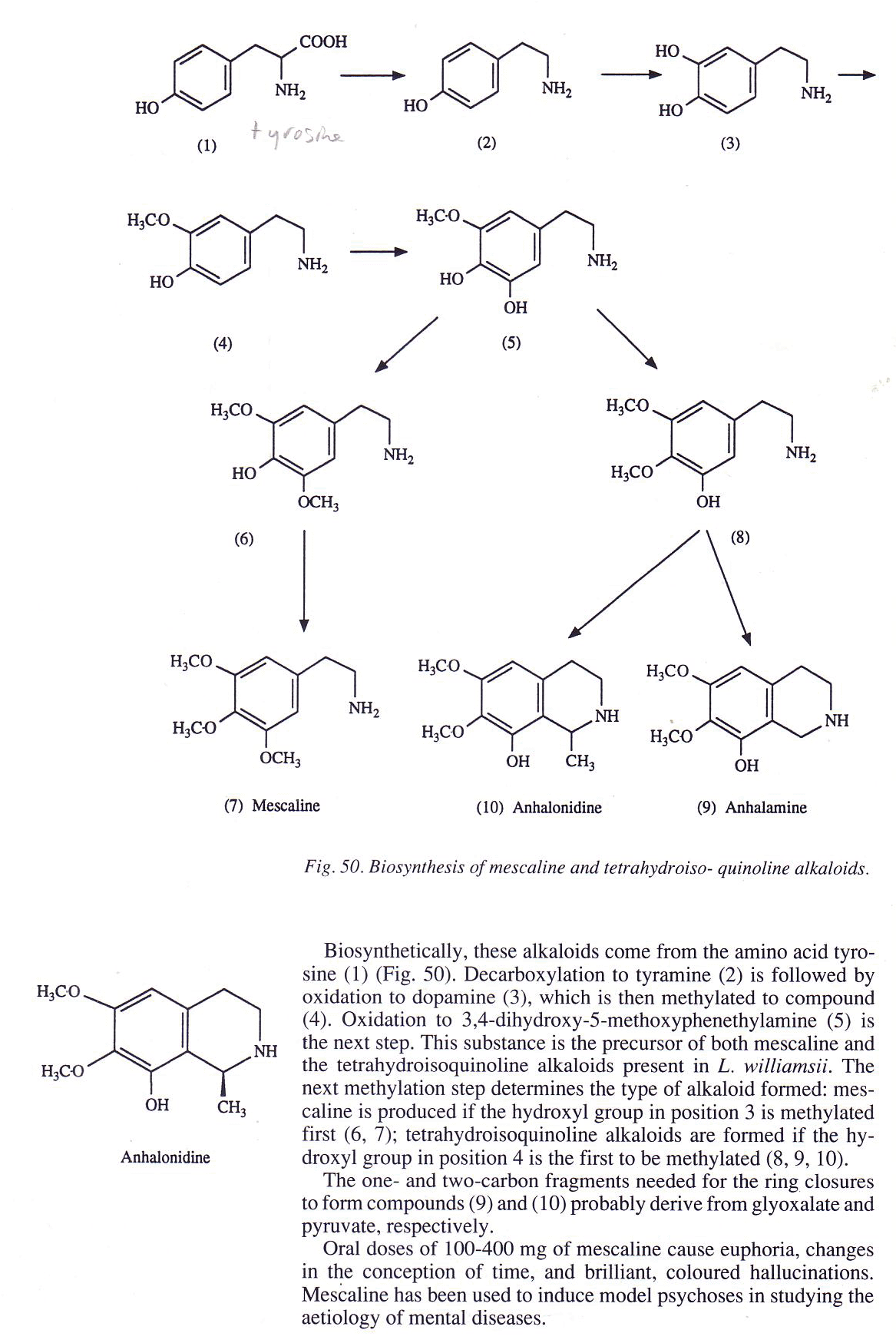
Taken from: Drugs of Natural Origin (A textbook of pharmacognosy)
A little poison now and then: that makes for agreeable dreams.
(Newbee)
07-10-04 13:38
No 518532
Yes, the pathways are different but since dopamine is an intermediate in both of them they could be combined - tyrosine -> DOPA -> dopamine -> 3-O-methyldopamine -> 4,5-didesmethylmescaline -> 4-desmethylmescaline -> mescaline. He, and with some genetics - in vivo produced mescaline. But let`s stop dreaming up.
Something like answer to your question about the OHs: The O-C bond lengths of AM1 energy minimized shikimic acid are - C5 - 1.4215, C4 - 1.4185, C3 - 1.4205. The difference is almost insignificant. Maybe it`s the way Mother Nature does it. After all 3-OH is esterified and 5-OH is etherified - don`t you see the idea?
PS: Bond lengths are estimated values, I don`t bet they`re the real ones.