(Stranger)
09-03-01 20:25
No 209772
(Rated as: excellent)
I would like to rise the question of preparation of 2,5-dimethoxybenzaldehyde from hydroquinone again. It was discussed a month ago in „2C-X 'cursors from hydroquinon" thread and commented by Rh. and Os.
We all know the JCS Perkin I, 1980, 1862 article, witch describes the reaction of phenol (1,0 mol), SnCl4 (0,1 mol), Bu3N (0,4 mol) and paraformaldehyde (2,2 mol) in toluene (typo in original article, 200 ml should be 2000 ml) under nitrogen atmosphere and heating at 100°C for 8 h.
Patent US4151201 by the same author teaches this reaction to be performed with SnCl2 or SnCl4, 3 mol paraformaldehyde and reflux temperature (up to 110°C, 8h). Yield for 2,5-dihydroxybenzaldehyde is given 48%.
A new variation of this formylation reaction for production of 3-substituated salicylaldehyde derivatives is described in Patent US6080895 by Japanese authors, claiming yields of at least 95% !
This improved process comprises in first stage heating the phenol (1,0 mol), SnCl4 or SnCl2 (0,1 mol), a base (2,6-lutidine, Bu3N, Et3N etc., 0,92 mol) and paraformaldehyde (2,2 mol) in toluene (2160 ml) at 60-85°C until achieving conversion of 30 to 80% and then completing the reaction at 95-105°C (aprox. 10 hours).
For 2-hydroxy-3-methylbenzaldehyde from o-cresol 99% yield and 99% selectivity is given when the reaction is carried out at 80°C for 5h (1st stage) and then at 100°C for 10 h (2nd stage). However, the yield and selectivity drop to 42% and 75% respectively, when the reaction conditions are altered to 40°C for 7h (1st stage) and then at 95°C for 13 h (2nd stage).
But, when really performing this reaction with hydroquinone, that is almost completely insoluble in toluene and, even with careful heating in an oil bath and strong overhead stirring, the entire reaction mass will polymerise already at 70°C, yielding beautiful resin and only a trace amount of 2,5-DMB. It is obvious that proper reaction conditions are essential to avoid thermal degradation and polymerisation of hydroquinone.
Can anybody report a better experience with this reaction?
(Chief Bee)
09-03-01 21:30
No 209786
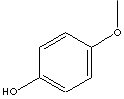
Wouldn't the solubility/polymerization thing be resolved if 4-methoxyphenol was used instead of hydroquinone?
(Daddy)
09-04-01 13:15
No 209960
Cesium, did you perform the reaction under a nitrogen atmosphere?
Safety data for 4-Methoxyphenol (a.k.a.hydroquinone monomethyl ether):
http://physchem.ox.ac.uk/MSDS/M/4-methox
Stable; incompatible with halogens, oxidizing agents.
Melting point: 55-57 C
Boiling point: 243 - 246 C
Solubility: Slightly soluble in water; soluble in organic solvents such as alcohol, ether and benzene.
Incompatibilities: Contact between 4-methoxyphenol and oxidizing agents, strong bases, acid chlorides, or acid anhydrides may cause reactions to occur. (like p-methoxyphenol +KOH ---> potassium p-methoxyphenoxide)
Hydroquinone and certain derivatives are also used as polymerization inhibitors by direct reacting with peroxy-free radical to tie up free radicals.
+++++++++++++++
Why not use this one:
2-Tert-Butyl-4-Methoxyphenol C11 H16 O2
OH
| \ /
| \/
/\\ /\
/ \\/ \
|| |
|| |
\ //
\//
|
|
O
\
\
The shelf life of margarine and other fats and oils is improved if oxygen attack on the double bonds in the carbon chains can be repelled or at least diverted, for then they do not turn rancid. The compound 2-tert-butyl-4-methoxyphenol or, more succinctly, BHA is an antioxidant, a substance that inhibits oxidation; it acts by interupting the chain reaction in which oxygen combines with double bonds and slices molecules in two. It does so by combining with peroxide *radicals (radicals of the form X-O-O, where X is th rest of the molecule) before they have time to attack other molecules and continue the chain.
Antioxidants have been used in foods for thousands of years, but their rules has only recently been appreciated. Among the more familiar ones are spices, which not only mask unpleasant odors but also help to prevent their formation. Sage, cloves, rosemary, and thyme all contain *phenolic compounds resembling BHA, which interrupt the chain reactions and stabilize fats against oxidation. Thyme oil is also effective against bacteria and has been used in gargles, mouthwashes, and disinfectants. Animals contain antioxidant materials, including vitamin E, that serve a similar purpose - to stop them from going rancid while they are still alive.
Sorry, I like pics. LT/
WISDOMwillWIN
(Daddy)
09-04-01 14:01
No 209976
Can't you do nothing with eugenol, 4 Allyl-2-methoxyphenol ?
Just found a source: ""Total production capacity is 50 ton per month. The product is packaged by coated drum and content 200 kg.
Light yellow liquid, oil. Spicy odor and taste, 98% purity by GLC test, technical grade.
APPLICATIONS: Medicine, Flavoring, Dentistry, Perfumery, Confectionery, Soap, plus conversion to Isoeugenol and then Vanillin.""
WISDOMwillWIN
(Daddy)
09-04-01 14:24
No 209987
Catechol production by O-demethylation of 2-methoxyphenol using the
obligate anaerobe, Acetobacterium woodii: M.S. Kalil, G.M. Stephens, PP.
1165-1168 Biotechnology Letters ISSN: 0141-5492 LT/
WISDOMwillWIN
(Daddy)
09-04-01 14:59
No 209996
Ahh, nearly forget to mention:
The reaction of 4-methoxyphenol with 1-chloro-3-methyl-2-butene gave the ether which underwent the thermal Claisen rearrangement to 2-(1,1-dimethylallyl)-4-methoxyphenol with a bp of 148-157 °C at 30 mm/Hg. This was cyclized to the intermediate cycle 2,3,3-trimethyl-2,3-dihydrobenzofuran which, after distillation, was shown to be only 80% pure by GC analysis. This was, nonetheless, (and with the hope that is in the very fiber of a young innocent chemist), pushed on to the benzaldehyde stage (and there were a not-too-surprising four benzaldehydes to be found in the oil that was produced, which refused to crystallize). And then (when sheer desperation replaced hope) these were condensed with nitroethane to form an even worse mixture. Maybe something might crystallize from it? Nothing ever did. Junk. Everything was simply put on the shelf where it still rests today, and F-233, 6-(2-aminopropyl)-5-methoxy-2,3,3-trimet
From: http://www.erowid.org/library/books_onli
Erowid Online Texts : PiHKAL #80 F-22
Entry #80 F-22 from PiHKAL by Alexander & Ann Shulgin.
See also PIHKAL #167 and #79 F-2
LT/
WISDOMwillWIN
(LabChamp)
09-04-01 19:27
No 210052
Thanks Rh and LT for suggestions.
No, N2 atmosphere was omited, SWISC was to lazy :-((
(Stoni's sexual toy)
07-12-02 15:16
No 331694
This thing needs to go up again!
I'm not fat just horizontally disproportionate.