(Hive Bee)
12-24-02 19:58
No 392484
(Rated as: excellent)
Abstract/Intro:
Purpose of experimentation was to create, optimize, and standardize a microwave assisted one pot reaction to transform aldehydes into nitriles using hydroxylamine hydrochloride, phthalic anhydride, and N-methylmorpholine.
Procedure:
Place hydroxylamine hydrochloride (1.1 mmol), N-methylmorpholine (1.2 mmol), phenylacetaldehyde (1.0 mmol), and 1,2-dichloroethane (1-2 ml) in a concical flask, test tube, or beaker. Place the reaction vessel in an unmodified domestic microwave and irradiate at high power for 1 to 2 minutes or until all starting material is reacted into aldoximes. (This can be observed via TLC in a 3:7 solution of ethyl acetate:hexane). Then add the phthalic anhydride (1.2 mmol) and microwave for 3 to 4 minutes at high power, or until all the aldoxime is completely reacted (TLC in a 3:7 solution of EtOAc:Hexane). The product would then undergo a work up procedure consisting of dilute sodium hydroxide solution to remove phthalic acid completely and then potable water. After working up, sodium sulfate is added to the solution to remove water molecules; the solution was then filtered. The filtered solution was then evaporated under reduced pressure and then dried in a vacuum pump. Melting point tests and Infrared Spectrophotometric Analyses were performed to confirm the
product.
Conclusion:
Phenylacetaldehyde was converted to benzyl cyanide in 8 Minutes at the highest effect in a domestic microwave oven with 97% yield of the product.
Se also:
../rhodium /ampheta
Overall yield (to the amphetamine): 88.5 %
Reference:
Microwave Assisted Rapid Transformation of Aldehydes Into Nitriles by Frank P. De Paoli
"Turn on, Tune in and Drop Out"
(Hive Bee)
12-29-02 16:56
No 393842
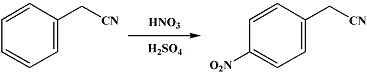
Procedure:
In a 2-l. round-bottomed flask, fitted with a stopper holding a dropping funnel and a mechanical stirrer, is placed a mixture of 275 cc. (4.3 moles) of concentrated nitric acid (sp. gr. 1.42) (Note 1) and 275 cc. (4.9 moles) of concentrated sulfuric acid (sp. gr. 1.84). This is cooled to 10° in a freezing mixture, and 100 g. (0.85 mole) of benzyl cyanide (free from alcohol and water) (Note 2) is run in slowly, at such a rate that the temperature remains at about 10° and does not exceed 20°. After all the benzyl cyanide has been added (about one hour), the ice bath is removed, and the mixture is stirred for one hour and then poured onto 1200 g. of crushed ice. A pasty mass slowly separates; more than half of this mass is p-nitrobenzyl cyanide, the other constituents being o-nitrobenzyl cyanide, and a variable amount of an oil which resists hydrolysis; apparently no dinitro compounds are formed. The mass is filtered on a porcelain funnel with suction, pressed well to remove as much oil as possible, and dissolved in 500 cc. of boiling 95 per cent alcohol. On cooling, p-nitrobenzyl cyanide crystallizes; the mother liquor, on distillation, gives an impure alcohol which can be used for the next run. Recrystallization from 550 cc. of 80 per cent alcohol (sp. gr. 0.86 to 0.87) yields 70–75 g. (50–54 per cent of the theoretical amount) (Note 3) of a product which melts at 115–116°.
http://www.orgsyn.org/orgsyn/orgsyn/prep
"Turn on, Tune in and Drop Out"