(Chief Bee)
10-22-03 09:46
No 466086
(Rated as: excellent)
Those who are more interested in the preparation of 48% aqueous HBr are referred to Post 188229 (lugh: "Six Laboratory Preparations of Hydrobromic Acid", Methods Discourse)
Triphenylphosphonium Bromide: A Convenient and Quantitative Source of Gaseous Hydrogen Bromide
A. Hercouet, M. Le Corre
Synthesis (2), 157-158 (1988)
Summary
Thermolysis of triphenylphosphonium bromide in refluxing xylene provides quantitative yield of anhydrous hydrogen bromide.
Utilization of a known quantity of hydrogen bromide is often necessary for many organic reactions such as addition on ethylenic or acetylenic compounds, opening of epoxides or lactones, synthesis of bromoacetals.1
While there are several methods for the generation of anhydrous hydrogen bromide in the laboratory, the most common source is from the reaction of bromine with tetraline.2 We now report a simple and convenient new method for the small scale (10 mmol to 1 mole) preparation of anhydrous hydrogen bromide. Because of the weak basicity of triphenylphosphine (pKa = 2.73),3 triphenylphosphonium bromide is unstable to thermolysis - losing hydrogen bromide on heating.
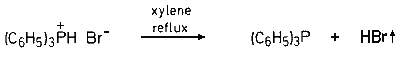
Simply warming the pure salt is not convenient, because variable yields (60-70%) of hydrogen bromide are obtained. We have observed, however, that the thermolysis in refluxing xylene is very clean and results in a quantitative yield of hydrogen bromide. A new method for the preparation of triphenylphosphonium bromide, which avoids the need to use gaseous hydrogen bromide, has also been developed. Although triphenylphosphonium bromide is destroyed by water, it can readily be prepared by extraction of a solution of triphenylphosphine in 48% aqueous hydrobromic acid with chloroform.
Since triphenylphosphine is recyclable, this procedure can be considered a preparation of anhydrous hydrogen bromide from its aqueous solution. The other possibilities offered by triphenylphosphonium bromide were described by Bestmann, et. al.4
Triphenylphosphonium Bromide
To 48% aq. HBr (350 ml.) is added Ph3P (131 g, 0.5 mol). After stirring at 70°C for 5 min, the solution is cooled and extracted with CHCl3 (3x150 mL). The combined organic phase is dried (Na2SO4), and the solvent is removed in vacuo. The residue is washed with warm EtOAc (300 mL) to remove traces of Ph3P; yield: 167 g (97%), mp 185-195°C/(Lit.5 mp 170-198°C, dec.)
Purity of this salt, as determined by acidimetric titration in a two-phase system H2O/CH2Cl2, is up to 99%.
Gaseous Hydrogen Bromide
In a 250 mL, two-necked, round bottomed flask equipped with a reflux condenser and a gas-inlet tube reaching to the bottom of the flask. are placed dry xylene (70 mL) and triphenylphosphonium bromide (34.6 g, 0.1 mol). A slow stream of nitrogen is introduced into the suspension to carry out HBr, and the reaction mixture is heated to reflux. After 30-40 min 99% of the theoretical quantity of acid is obtained.
Evaporation of the xylene allows the recovery of Ph3P.
References
[1] Stowell, J. C., Keith, D. R., King, B. T. Org. Synth. 62, 140 (1984) (http://www.orgsyn.org/orgsyn/prep.asp?p
[2] Vogel. A. Vogel's Textbook of Practical Organic Chemistry, 4th ed., Longman, London, p. 297 (1978)
[3] Henderson, W A., Streuli, C. A. J. Am. Chem. Soc. 82, 5791 (1960)
[4] Bestmann, H. J., Mott, L., Lienert, J. Liebigs Ann. Chem. 709, 105 (1967)
[5] Seyferth, D., Grim, S.O., Read, TO. J. Am. Chem. Soc. 83, 1617 (1961)
(Newbee)
11-03-03 23:24
No 468554
Triphenylphosphonium Bromide: A Convenient and Quantitative Source of Gaseous Hydrogen Bromide
A. Hercouet, M. Le Corre
Nice!
But SWIM can have HBr more easily.
HBr can be made from KBr by strong acids like H2SO4. The problem that H2SO4 is a very strong oxidizer the HBr will be oxidized to Br2. Instead of H2SO4 SWIM can use konz.H3PO4.
Another method if not anhydrous is needed SWIM can also use this reaktion
H2S + Br2 --> 2HBr + S
Look also : Post 468552 (politoxicomania: "Gas SWIM needs", Chemistry Discourse)