12-06-03 15:31
No 475089
I might be wrong, but can you..
1. React toluene with Jones reagent, or (eq oxidation), which would give you benzoic acid.
2. React pure benzoic acid with urea to give benzyl acetamide.
3. React benzyl acetamide with phosphorus pentoxide to give the benzyl cyanide, which could be used to make P2P?
(Chief Bee)
12-06-03 17:49
No 475113
2. React pure benzoic acid with urea to give benzyl acetamide.
Benzoic acid and urea would give benzamide, not "benzyl acetamide". Now count the carbons in each of the molecules below. How would you get from one to the other?
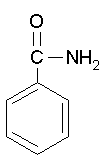
Benzamide
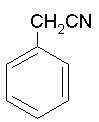
Benzyl Cyanide
(Hive Bee)
12-06-03 18:49
No 475117
I always thought that a primary amide could be dehydrated to a nitrile using P2O5, POCl3, or SOCL2...................................
12-06-03 18:54
(Rated as: ALL CAPS)
(Hive Bee)
12-07-03 01:35
No 475173
The way from toluene
Toluene -> Benzaldehyde -> Benzylamine -> Benzylcyanide
Post 453906 (roger2003: "Benzylcyanide", Methods Discourse):
roger2003