01-03-04 10:52
No 480268
Ok I just talked to an aquaintance of mine who expressed interest to me in synthing and trying bzp. He told me he has a rather rudimentary lab at this point in time. (He does have a vac pump now though thank god!)
Looking at the synth on Rhodium's site he wonders about benzyl chloride because he doesn't have any way to distill anything! He does have LG toulene and has enough glassware to make a rig to produce chlorine (and HCl gas when needed later) but doesn't know if freshly synthed benzyl chloride is acceptable for this synthesis, or if it needs to be distilled?
My friend has obtained 100g of lg piperazine (the ethanesulfonic acid salt). Here is the personalized procedure that he has relayed to me.
-Take 50g Piperazine ethanesulfonic acid
-Add NaOH in small portions until ph of 7 is achieved
-Dry and weigh resulting freebase
-Prepare a solution of 24.3g piperazine freebase in 50ml absolute ethanol in a 250ml flask
-Warm in bath to 65C
-Swirl in 22.1g of the dihydrogenchloride salt
produced by the following from rhodium's site.
A brisk stream of hydrogen chloride gas is passed for 5-8 minutes into a solution of 24.3g (0.125 mole) of piperazine hexahydrate in 50 ml of absolute ethanol contained in a 250-ml Erlenmeyer flask. A wide gas-inlet tube (about 10mm) is used to avoid clogging, and the flask is cooled in an ice bath to keep the temperature at about 25°C. After the gas stream has been discontinued, the contents of the flask are cooled to about 0°C, and the crystalline product is collected by suction filtration and washed with two 25ml portions of ice-cold absolute ethanol. The yield is about 22g (0.125 mole).
-Add during a period of 5 minutes, 15g of benzyl chloride (says freshly distilled, this is where his question is, can he use freshly synthed and not distilled benzyl chloride?), under vigorous stirring.
-Continue stirring for add't 25minutes at 65C
- Remove from heat
- Put in icebath for 30 minutes
- crystals of piperazine dihydrochloride monohydrate are collected by suction filtration, washed with three 10ml portions of ice-cold absolute ethanol, and then dried. Recovery of the dihydrochloride is 21.5-22.0 g. (97-99%)
-The combined filtrate and washings from the piperazine dihydrochloride are cooled in an ice bath and treated with 25 ml of absolute ethanol saturated at 0°C with dry hydrogen chloride (Note 3). After the solution has been well mixed, it is cooled for 10-15 minutes in an ice bath. The precipitated white plates of 1-benzylpiperazine dihydrochloride are collected by suction filtration, washed with dry benzene, and dried. The product, which melts at about 280°C with decomposition, after sintering at about 254°C (Note 4), amounts to 29-29.5g. (93-95%).
-A solution of this salt in 50ml of water is made alkaline (pH > 12) with about 60 ml of 5N sodium hydroxide, then extracted twelve times with 20ml portions (Note 5) of chloroform. The combined extracts are dried over anhydrous sodium sulfate, and the pale brown oil (Note 6) remaining after removal of solvent is distilled at reduced pres in a Claisen flask. The yield of pure 1-benzylpiperazine, bp 122-124°C/2.5 mmHg, is 14.3-16.5 g. (65-75%)
(Chief Bee)
01-03-04 15:19
No 480312
he doesn't have any way to distill anything!
Then his first objective is to aquire a suitable distillation setup before trying to synthesize anything which is to be ingested. If he doesn't have the means to distill anything, then how is he going to remove potentially toxic impurities and byproducts?
The Hive - Clandestine Chemists Without Borders
(Stranger)
01-03-04 19:13
No 480332
He relayed to me that he does have a suitable distillation setup on order, he was just desiring to play around with the piperazine he came across while he was waiting, an impatient friend I guess. Thank you for enlightening my friend. He does have one further question, to produce the piperazine hexahydrate from the ethanesulfonic acid salt all he would need to do is base it correct?
(Chief Bee)
01-03-04 19:47
No 480335
Yes, base it and then recrystallize it from water. The piperazine hexahydrate will form nice, large crystals.
The Hive - Clandestine Chemists Without Borders
(One Remarkable HyperLab Bee)
01-04-04 05:07
No 480358
My friend has obtained 100g of lg piperazine (the ethanesulfonic acid salt).
There is no commercial reason to produce the ethanesulfonate salt of piperazine. So, I guess that your friend has a bottle of PIPES (piperazine-N,N'-bis(2-ethanesulfonic acid), 1,4-piperazinediethanesulfonic acid, piperazine-1,4-bis(2-ethanesulfonic acid) - there may be some more alternative names). This compound is not a piperazine salt. The ethanesulfonic acid moieties form covalent bonds with the piperazine nitrogens:
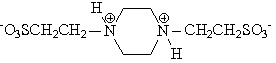
(Stranger)
01-04-04 11:05
No 480378
Yes I suppose he should have been a bit more clear. It is the PIPES you speak of, my friend told me it says it PIPES clearly on the bottle, he just wrote it off when he saw it. He did tell me it is used to prepare buffer solutions as the alfa aesar (not really a source is it? :) ) catalog stated. So this 'PIPES' is essentially useless in the quest my friend has undertaken
(Newbee)
01-04-04 11:50
No 480382
(Rated as: excellent)
Piperazine hydrochloride or more usually citrate of veterinary grade (used as a vermicide) is cheap and good enough for your goal.
If you get the citrate (that contains 36% piperazine base) you can transform it in to the hydrochloride (why you need that I’ll explain later):
Put 100g of piperazine citrate in a 500ml beaker and add 100ml of 30% hydrochloric acid (20 to 36% conc. will do if you recalculate the volume). Then add 100ml of ethanol (or isopropanol) and start heating on a water bath at ~60°C with frequent stirring for 30 minutes (the solids never completely dissolve, the just change crystal form). Let it cool at room temperature and dilute with acetone to a volume of ~500ml and suction filter the white crystalline solids. Wash them with acetone a few times and let them air dry. The yield of piperazine dihydrochloride monohydrate is usually more than 90%.
From piperazine dihydrochloride monohydrate you can form an ethanolic solution of piperazine monohydrochloride that can be used for the alkylation with benzylchloride:
In a 500ml beaker dissolve 23g of sodium hydroxide in 250ml of ethanol with mixing at a temperature of ~60°C. While still hot slowly add 100g of piperazine dihydrochloride monohydrate and stir for an hour and then let it cool at room temperature. Then suction filter the solids (NaCl) and wash them thoroughly at least three times with ~70ml of ethanol. The combined filtrates are then concentrated to a volume necessary to get the concentration of piperazine monohydrochloride to above 2 mol/L (V<280ml). A vacuum pump is a good idea but merely distilling off the ethanol will do it. The volume of the warm solution is measured accurately and the concentration of piperazine monohydrochloride is calculated based on the 0.565mol of piperazine dihydrochloride monohydrate used at the beginning. The solution is over saturated so warming is necessary prior any use to dissolve the crystaline piperazine monohydrochloride.
Now you have the ethanolic solution that can be used in the known BZP synthesis. This procedure avoids the tedious isolation of the hygroscopic piperazine hexahydrate - it is highly water soluble, very hygroscopic and you can never be absolutely sure about the piperazine content.
The raw product BZP monohydrochloride can be recrystallized from isopropanole. If you don’t like the smell of it you can form the odorless and more nicely cristaline BZP dihidrochloride by dripping an molar equivalent of conc. hydrochloric acid to the suspension of BZP monohydrochloride in izopropanol (or acetone), mixing it for 30min and filtering it off.
The procedure from Org. Synth. (also on Rhodium’s site) does yields almost only the monoalkylated product even in the raw product (the hydrochloride functioning as a protecting group and the precipitating dihydrochloride making sure the piperazine in solution is always a monobasic salt). But in case anybody is concerned about the possible contamination with N,N’-dibenzylpiperazine I can confirm it does not have any noteworthy acute toxicity up to doses of 300mg (as a dihydrochloride) though I don’t know about any possible chronic toxicity. Since the yield of the above preparation of the ethanolic solution is only assumed to be 100%, assuming it to be 95% might be better, thus contaminating the product with the harmless piperazine hydrochlorides instead of the less known dibenzylpiperazine. In any case the piperazine dihydrochloride will be left over while recrystallising the product from isopropanole. It will however take some hydrochloric acid from the BZP monohydrochloride thus somewhat lowering the yield of the recrystallisation.
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Stranger)
04-17-04 07:47
No 501288
nicodem, how much benzyl chloride is added after Ethanolic Piperazine Monohydrochloride is prepared from 100g of piperazine dihydrochloride?