01-15-04 09:52
No 482599
Are there any commercial HI Gas traps/Vapor containment Units available for SWIM to purchase? SWIM wants to use something more effective than a balloon for 5g ephedrine reductions?
Assuming swims budget is 1K USD for a gas trap/VPU? What would fellow bees suggest? SWIM lives in an apartment complex, SWIM wants absolutely no smells? How about the charcoal carbon filters used in weed-ops? Will those work here? Any other suggestions?
(Chief Bee)
01-15-04 15:59
No 482664
(Rated as: good read)
There are thousands of posts available in the archive on smell/fume absorbation/elimination.
My personal favorite for acidic fumes is to fill a gas wash bottle with sodium carbonate and activated carbon, starting with ~5cm activated carbon at the bottom of the flask where the inlet tube opening is, and then alternating 2-3 cm thick layers of granular sodium carbonate (washing soda) and coarse activated carbon all the way to the top.
This absorbs all acidic gas evolved from a reaction as long as the flow isn't very rapid. It's excellent for thionyl chloride chlorinations, Friedel-Crafts alkylations and Vilsmeyer formylations in my own experience, and it can be used for at least a molar batch of any of the above reactions without any need for recharging.
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
01-15-04 18:23
No 482707
(Rated as: excellent)
Gas Absorption Trap
Organic Syntheses, CV 2, p. 3
A convenient trap devised by John R. Johnson for the absorption of hydrogen chloride, or for the elimination of sulfur dioxide, hydrogen cyanide, etc., may be arranged as shown in the figure.
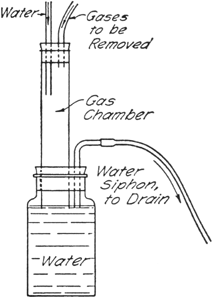
The gases are led into a chamber in which a stream of water (from the reflux condenser in this case) flows downward into a large bottle. The bottle is provided with a bent tube which serves as a siphon drain. The gases are thus brought into contact with a flowing stream of water so that the heat of solution is dissipated, and the level of the water in the lower bottle serves as a seal to prevent escape of the gases into the atmosphere. Water-insoluble gases are drawn out through the siphon drain directly into the sink.
If the gas chamber is of sufficient capacity there is practically no danger of water being drawn back into the reaction vessel, but care should be exercised when the reaction flask is cooled. For the reaction described here, a gas chamber about 2.5 cm. in diameter and 20–25 cm. in length was found to be satisfactory.
In reactions involving compounds sensitive to water it is desirable to introduce a drying tube between the condenser and the trap in order to absorb any moisture which might diffuse up from the trap.
An alternative, and simpler, gas absorption trap is described in Organic Syntheses, CV 1, p 95:

The Hive - Clandestine Chemists Without Borders
(Hive Bee)
01-15-04 19:20
No 482726
Thanks Rhodium. The Gas absorption trap info is great. SWIM will get the necessary material to make the trap. Would this trap eliminate all gases created during reflux of 5g eph/hypo/I2 ?