03-24-04 05:46
No 497031
Ok I am new... non chemist. Don't know all the bee-speak so I apologize in advance. My question is is there a way to remove a methyl from a ketone and replace it with a benzene? if i knew how to use this board i would paste my equation theory. I realize this is probably a stupid question... but i need to learn somewhere.
(Hive Bee)
03-24-04 06:18
No 497038
There is a way to cleave of a methyl group from a ketone through the haloform reaction,
Et-CO-Me ---> EtCOOH + HCX3
but there 's no way to chop off the beta methyl and replace it by a benzene.
realize this is probably a stupid question... but i need to learn somewhere.
I wish you good luck exploring the field of organic chemistry. You'll see it is a fascinating world. A good way to start learning is to buy an (elementary) organic chemistry is to buy a textbook, like:
Solomons, Organic chemistry
(Hive Bee)
03-24-04 08:52
No 497058
Dimethyl Ketone (acetone) can be condensed with benzene yielding phenylacetone. Look up Friedel Crafts reaction. The workup on this method is a real mess and the yields not so great but only one step unless you make the chloroacetone yourself.
(Chief Bee)
03-24-04 12:30
No 497079
And there is of course this method of producing P2P, which uses methyl ethyl ketone as a starting material: Post 245942 (twodogs: "New method for P2P", Novel Discourse)
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
03-24-04 14:14
No 497095
That is a rather neat synthesis
Unfortunately, working with peracids has its inherent dangours. m-chloroperbenzoic acid may bee one of the safer compounds to use (more stable).
(Hive Bee)
03-24-04 20:43
No 497177
The perborate in the twodogs procedure produces the per acid in-situ so it is quite safe as long as the exotherm is kept under control. The tetrahydrate form is very stable and will lose only 10% a year active O2 according to the maker. The monohydrate is more active but much less stable. The persalts in this reaction give about the same results as pre made peracid.
(Stranger)
03-25-04 06:16
No 497263
ok I am a little enlightened. Several hours sourcing and I am wondering what if anything makes this an unattractive synth? Is it the poor yield (30%)?
(Chief Bee)
03-25-04 06:28
No 497266
Yes. And also the fact that it isn't very well-known.
The Hive - Clandestine Chemists Without Borders
(Stranger)
03-25-04 08:12
No 497278
I am not a chemist, but have a bio background... unfortunately years ago. this is starting to look like a dream i have about catalytic antibodies replacing hard to get catalysts. the dream goes something like this... we need compound A (benzaldehyde} and Compound B (MEK) to be held in space in such a way that the activation energy goes to ~0 and specificity (ie no side reactions)is ~100%. In this dream we need to stabilize the transition state Of Benzaldehyde + MEK = p2p (actually i see no reason that benzene + MEK can't be employed if a catalytic antibody can in fact be created). To stabilize transition state we need an analog of the transition state (this is for you chemists... i don't even have a clue in my dream) if we know this analog it might be possible to locate an abzyme in literature that will work.
Benefits:
Extremely high yields and reaction times
fairly easy to produce (if in fact one exists)
extremely low ratio of catalyst to reactants
i know this is a fucked up post. any and all criticisms including flames welcome.. i learn the hard way always.
alpha
(Chief Bee)
03-25-04 08:37
No 497283
It is too complicated to be performed like that, it is in fact a three-step procedure:
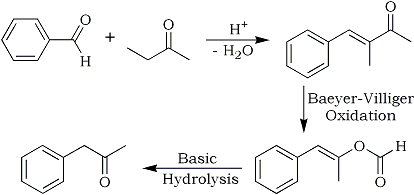
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
04-02-04 17:00
No 498766
Benzaldehyde is easier to get than one would imagine especially if you are taking the appropriate precuations when ordering. The other chems are cake to obtain. This method works, and is about as OTC as one would hope to achieve. The yields seem low..but careful examination of the original patents and literature will uncover *possibilities* to increase yield to 95%. Obvious ones after limited experience include.. 1. Isolating the sodium borate after the baeyer-villiger step..and recharging with fresh sodium PERborate and allowing the reaction to procede again before separating the ester. 2. Manipulation of the temperature/time/unsat. ketone ratios to: peracid allowed for the baeyer-villiger step. 3. Strength/amount of mixture used to saponify the obtained enol ester.
Read the literature that is out there on the baeyer-villiger..its not a new reaction..and there are a lot of possibilities out there in ways of catalysts etc.
It works.
The shortest distance between two points is a straight line