(Stranger)
06-10-04 21:05
No 512616
(Rated as: excellent)
The hydrogenation of a carbon-carbon double bond
The simplest example of this is the reaction between ethene and hydrogen in the presence of a nickel catalyst.

In practice, this is a pointless reaction, because you are converting the extremely useful ethene into the relatively useless ethane. However, the same reaction will happen with any compound containing a carbon-carbon double bond.
One important industrial use is in the hydrogenation of vegetable oils to make margarine, which also involves reacting a carbon-carbon double bond in the vegetable oil with hydrogen in the presence of a nickel catalyst.
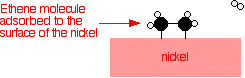
Ethene molecules are adsorbed on the surface of the nickel. The double bond between the carbon atoms breaks and the electrons are used to bond it to the nickel surface.
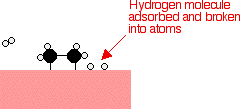
Hydrogen molecules are also adsorbed on to the surface of the nickel. When this happens, the hydrogen molecules are broken into atoms. These can move around on the surface of the nickel.
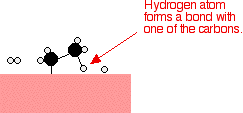
If a hydrogen atom diffuses close to one of the bonded carbons, the bond between the carbon and the nickel is replaced by one between the carbon and hydrogen.
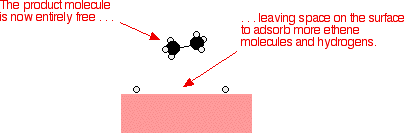
That end of the original ethene now breaks free of the surface, and eventually the same thing will happen at the other end.
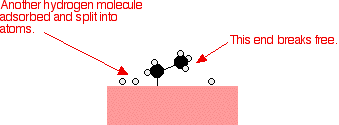
As before, one of the hydrogen atoms forms a bond with the carbon, and that end also breaks free. There is now space on the surface of the nickel for new reactant molecules to go through the whole process again.
http://www.chemguide.co.uk/physical/cata