09-06-04 09:28
No 529854
../rhodium /4-meo-p
In the above outline, the author mentions that substituting piperidine for pyrrolidine would create an end product equally as potent as 4-Methoxy-PCP without passing through the Schedule 2 substance PCC. If such a substitution were made, what would the new intermediate product be? What would the resulting product be? Would any other changes need to be made to the synthesis?
(Chief Bee)
09-06-04 12:09
No 529926
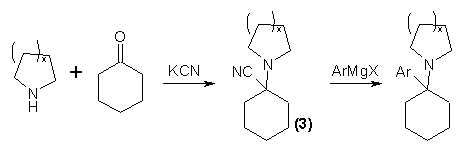
The intermediate would look like compound #3, and the product like the rightmost substance. The "ArMgX" is 4-Methoxy-phenylmagnesium bromide in your case, so the "Ar-" of the product would be a 4-Methoxyphenyl ring.
Ignore the ( )x in the picture - it's irrelevant.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
09-06-04 16:09
No 529992
1. Do you believe that the author is correct in stating the end product would be equally as potent as 4-Methoxy-PCP?
2. What software did you use to render these images?
(Chief Bee)
09-06-04 20:28
No 530044
1. Do you believe that the author is correct in stating the end product would be equally as potent as 4-Methoxy-PCP?
Yes, you can read up on general structure-activity relationships here:
../rhodium /pcp.shu
../rhodium /clandes
2. What software did you use to render these images?
Post 461238 (Rhodium: "ISIS-Draw vs. ChemOffice", Newbee Forum)
Post 466087 (Rhodium: "ISIS -> GIF in Photoshop", General Discourse)
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
09-07-04 00:10
No 530074
First off, the author of the synthesis claims 4-Methoxy-PCP to posses a potency of about 70% of PCP's, while Table IV in the second article you listed claims it to have a potency of 10% of PCP's; a serious discrepancy. Does anyone have enough experience to give a third opinion? Also, after reading the text, I believe that the author of the synthesis may also have been wrong in that the piperidine substitution would not yield a product equally potent or even with the same qualitative effects. It would be disappointing to go through all the trouble of creating such a compound only to find it has a "street use to cause a barbiturate-like sedation." Do you agree with my interpretation?
(Chief Bee)
09-07-04 00:51
No 530081
First off, the author of the synthesis claims 4-Methoxy-PCP to posses a potency of about 70% of PCP's, while Table IV in the second article you listed claims it to have a potency of 10% of PCP's; a serious discrepancy
No, that's the wrong substituent on the wrong ring...
Table IV: PCP with a Methyl group in the 4-position of the piperidine ring.
4-meo-pcp.html: PCP with a Methoxy group in the 4-position of the benzene ring.
The Hive - Clandestine Chemists Without Borders