12-28-01 13:11
No 250931
US Pat. #4,568,700
Chloromethylation of aromatic substrates and polymers is carried out readily using chloromethylether (CME) or bis-chloromethyl ether (BCME). Since both reagents have been listed as highly carcinogenic, the alternative use of formaldehyde or paraformaldehyde in acidic aqueous solutions has been tried. This method was found unsafe, since BCME is formed under those condtions, and acts as the active chloromethylating intermediate. The same holds true for other methods using formaldehyde derivatives. The major hazard in the use of CME and BCME lies in their high volatility which causes lung cancer
We have now discovered, that long chain alkyl-halomethyl ethers are very effective and safe halomethylating agents.
Preparation:
0.1 mole of the alcohol and 0.1 mole of paraformaldehyde, and 10 gr of a drying agent (CaCl2) were suspended in 100 ml 1.1.2.trichloroethane (several haloalkanes (e.g., CHCl3,) were used actually). The stirred mixture was cooled to 0-5 C. and HCl gas was introduced for 2 hours. An aliquot from the liquid phase was taken and analyzed by N.M.R. (after dilution with CDCl3) and by combined gas-chromatography/mass spectrometry (70 C. for 3 min, then temperature increase 6/min to 170 C.
Alcohol % ROCH2Cl % BCME
n-heptyl 56 <10-6
n-decyl 52 <10-6
n-dodecyl 100 <10-6
n-tetradecyl 35 <10-6
Oleyl 50 <10-4
2-octyl 52 <0.003
cyclohexyl 58 0.025
menthyl 80
o-nitrophenyl 50 0.06
Glucose 25 0.07
In the case of primary alcohols, the ratio of alcohol-to-CH2O units determines the presence of BCME, a slight excess of ROH is needed to ensure the total absence of BCME. If a small amount of polyhydric alcohol, such as glucose, is added, then no excess of the primary alcohol is needed.
Example:
Chloromethylcyclohexylether
30 g (0.1 mole) of paraformaldehyde were stirred in 100 ml 1,1,2-trichloroethane, at 0.degree.-5.degree. C. with 90 g (0.1 mole) of cyclohexanol. HCl gas was passed for 2 hours. The chloromethylcyclohexyl ether was diluted to 0.2M concentration.
Any primary or secondary alcohol >6C atoms, or even menthol or glucose can bee used - they will probably (no examples) bee as effective chloromethylation agents - at least, polychloromethylated polymers themselves work too
Chloromethylation of toluene
9.2 g (0.1 mole) toluene were reacted at 0-5 C. with 100 ml 3M C8 H17 OCH2 Cl in 1,1,2-trichloroethane in the presence of 1.0 ml SnCl4 (in many patents, ferrric chloride is used instead in almost identical cond's with success) for 4 hours. After treatment of the solution with 20 ml HCl and water, the residue was analysed by MS-G.C. (see example 7) and was found to contain a mixture of para and ortho products in the ratio of 2.5:1. The degree of conversion was 92%.
Unfortunately, one canít chloromethylate (at least, bromomethylate) veratrol w/this proc. Ė duh, one should have expected it.
One-step chloromethylation (or halomethylation) can be achieved in a system containing the following components:
1. a transition metal halide as a source for HCl (or HX), e.g. TiCl4, ZnCl2 in equimolar amount.
2. equimolar amount of paraformaldehyde.
3. a catalytic amount of ROH
4. a Lewis acid catalyst, i.e. SnCl4.
unfortunately, tested only on polymers.
Destruction of reagent with hexamethylene tetramine (urotropin)
10 ml (20 mmole) of 2M C.sub.8 H.sub.17 OCH.sub.2 Cl were reacted with 3.1 g (22 mmole) C.sub.4 H.sub.8 N.sub.4 (hexamethylene tetraamine). The reaction mixture heated spontaneously and a crystalline product (6.7 g) separated out.
The product is scarcely soluble in H.sub.2 O, CH.sub.3 OH, CHCl.sub.3, or CH.sub.3 SOCH.sub.3. The solid was washed with chloroform, and the solution was found by NMR to contain only n-octanol, indicating a quantitative destruction of the reagent to yield (N-methyloxyoctyl) hexamethylene chloride. Further hydrolysis of this salt with acid yields n-octanol.
other possible neutralization reagents include methanol, water, amines
Antoncho
(Official Hive Translator)
12-28-01 14:03
No 250939
Chloromethylation of dichlorobenzene:
US Pat. #3217048
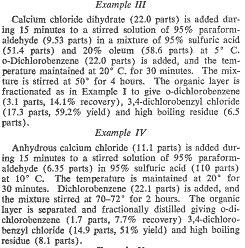
So... p-diCl-B is OTC, AFAIK... Can aromatic chlorines bee exchanged for -OCH3, you think? (After Sommelet to convert BzCl to PhCHO)
p-diBr-B can bee made...
Another crazy hypothetical route to p-diMeO-BA...
Antoncho
(Chief Bee)
12-28-01 18:22
No 250965
Us Patent 4,740,636 is an up to date version of the first patent you posted, but I have no idea what they have changed.