06-05-02 09:06
No 317862
piperine can be isolated from pepper and converted to piperic acid.
over 130 years ago (!) in 1869 fittig mielck described very detailed
[1](page 47-56) the cleavage of piperic acid to a compund they called
mono-bromo-piperonal. they wrote: "the preparation of this compund is
extremely easy and we obtained it each time in considerable quantity ..."
they used for the simple preparation: bromine, Na2CO3 and alcohol.
furthermore they performed extensive investigations on this compund,
beside other results, they found that mono-bromo-piperonal is extremely
easy volatile with steam, insoluble in cold water and sparsely in boiling
water. it crystallizes from alcohol in long, bendable and completely
colorless and glittery needles and melts at 129°C to a colorless liquid.
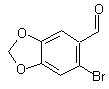
the molecular formula is C8H5BrO3.
[1] analen der chemie und pharmacie, 1869 152, 25-58
"untersuchungen ueber die constitution des piperins
und seiner spaltproducte piperinsaeure und piperidin"
rud fittig, w h mielck
in the paper are no particulars about the structure of mono-bromo-piperonal.
but piperonal has only 3 possible positions for a ring substitution
with bromine. so lets see what the internet nowadays knows about this
compund:
6-bromo-piperonal
=================
Molecular Formula: C8H5BrO3
Molecular Weight: 229.03
CAS Number: 15930-53-7
Air sensitive, Store under nitrogen
Melting Point (°C): 128 to 132
6-BROMO-1,3-BENZODIOXOLE-5-CARBOXALDEHYD
========================================
Molecular Formula: C8H5BrO3
CAS Number: 15930-53-7
[http://www.sigmaaldrich.com/]
[http://www.interchim.com/interchim/chemi
BINGO !!!
so it looks like mono-bromo-piperonal and 6-bromo-piperonal
are the same.
and 6-bromo-piperonal again is a precursor for:
MMDA-2 (http://www.erowid.org/library/books_onl
(Newbee)
06-06-02 04:57
No 318212
This should be rated as "excellent"!
Easy, high yielding sythesis. A valuable precursor for some NOT-SCHEDULED analogs (at least here in Germany!).
(Chief Bee)
06-06-02 06:28
No 318230
3base: You don't have access to the paper in question so that the actual procedure can be had?
(Official Hive Translator)
06-06-02 09:43
No 318277
Absolutely cool, 3base! I mean, fucking excellent! You made my day
So..... now we have a fully functional 'piperine routeset' analogous to the one that already exists for vanillin
At least, we will have it if the bromination procedure is found in explicit form
Only you, naturally, will 1st have to isolate piperine from pepper - but that's easy.
Another interesting mooment - they say bromopiperonal is very easy to steam-distill; makes me kinda wonder if the same rule would also apply to bromo-p-DMB
Antoncho
(Hive Bee)
10-08-02 11:59
No 366120
Here it is, someone please translate it.
Annalen der Chemie und Pharmacie 152, 25-58 (1869):
Die Darstellung dieser Verbindung ist aufserordentlich leicht, wenn
man auf die oben beschriebene Weise 1 Mol. Piperinsäure mit 2 bis 3 Mol.
Brom zusammenbringt, das product in heifsem Alkohol löst, nach dem Erkalten
die abgeschiedene Piperinsäure abfiltrirt, darauf den Alkohol verdunstet
und den harzigen Rückstand mit kohlensaurem Natrium und Wasser so lange
destillirt, bis sich im Kühlrohr keine Krystalle mehr verdichten. Die so
erhaltene Verbindung ist nahezu rein. Um sie vollständiger zu reinigen kann
man sie nochmals mit Wasser destilliren oder aus siedendem Alkohol oder
Benzol umkrystallisiren.
Die Analyse ergab für diese Verbindung die Formel C8H5BrO3. Sie ist
demnach Monobrompiperonal.
Das Monobrompiperonal ist unlöslich in kaltem, etwas löslich in
siedendem Wasser, leicht löslich in heifsem alkohol, wenig löslich in kaltem.
Aus Alkohol krystallisirt es in langen, biegsamen, völlig farblosen und
glänzenden Nadeln. Aus siedendem Benzol, worin es leicht löslich ist, wurde
es in harten Tafeln erhalten. Es schmilzt bei 129° zu einer farblosen
Flüssigkeit, erstarrt wieder krystallinisch und verflüchtigt sich schon bei
70° merklich. Mit Wasserdämpfen ist die reine Verbindung so leicht flüchtig,
dafs sich enge Kühlröhren in sehr kurzer Zeit verstopfen.
(Chief Bee)
10-08-02 12:35
No 366140
2-3 moles of bromine is added to piperic acid in the manner described above, the product recrystallized from alcohol and after cooling the precipitated unreacted piperic acid is filtered off and the alcohol is evaporated to give a resin, which is distilled together with aqueous sodium carbonate until no more crystals are deposited in the condenser. The product is relatively pure, but can be purified further by another steam distillation or recrystallization from alcohol or benzene.
The product 6-bromopiperonal is insoluble in cold water, somewhat soluble in boiling water, very soluble in boiling alcohol, not much in cold alcohol. The compound crystallizes from alcohol in long, shiny colorless needles. The melting point is 129°C.
(Stranger)
10-13-02 14:05
No 368101
6-bromo-MDX is a rough ride from what I understand.
Kind of like T#19 I would suppose.
As for N-X-(2-Me0)-4,5-MDO-Amp, I believe Ann used the phrase, 'not the smoothest of drugs.'
However, I can only describe 2-MeO-3,4-MDO-Amp as "extremely uplifting" [in my own words]. Luckily, I found some acid that night and that kept me from getting too "high."
(Hive Bee)
12-09-02 15:23
No 387923
2-3 moles of bromine is added to piperic acid in the manner described above
Moo, if you still have the ref. what is the bromination procedure "described above"? SWIM assumes that it is conducted in alcohol, probably methanol, correct? SWIM wants to verify because brominating in haloalkanes leads to tetrabromopiperic acid according to 3-base's page. Thanks.
(Hive Bee)
12-09-02 16:20
No 387938
It's a good thing someone noticed that one. I'm sick of typing up something in a language I have trouble understanding even with the help of a dictionary and will scan the whole article.
(Chief Bee)
12-09-02 19:28
No 387996
I have uploaded it to ../rhodium/djvu /fittig.djvu
( DjVu plugin: http://www.djvu.com/download/?x=2&p=1&o=
(Hive Bee)
12-11-02 11:11
No 388475
Thanks very much!