(Stunning)
06-18-02 19:08
No 322750
(Rated as: excellent)
Look at this!
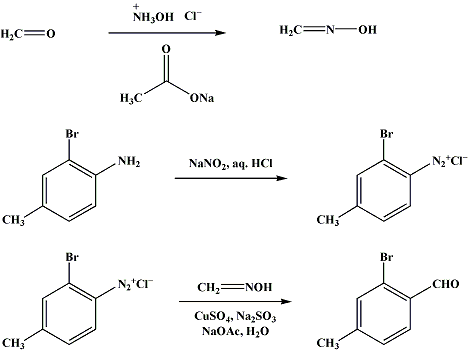
it's from http://www.orgsyn.org/orgsyn/prep.asp?rx
If one use 4-methyl-2,5-dimethoxyanilin (wich easy to produce by reduction of 4-nitro-2,5-benzaldehyde, wich produced by nitration of 2,5-DMBA) instead of 4-methyl-2-bromoanilin, finaly we produce 4-methyl-2,5-DMBA a direct precursor for DOM
SWIT also thinks it's easy way to some other pretty X-methyl-phenethylamines.
Matrix... may tricks?
(Stranger)
06-21-02 00:25
No 323489
Questions to experienced bees:
Is this reaction useful to methoxy (or methylenedioxy) aninlines?
if so:
if not:
(Stranger)
06-21-02 15:28
No 323638
Sorry,