(Official Hive Approximator)
07-21-02 11:27
No 335428
(Rated as: excellent)
this wonderful reference was found by foxy2.
if you want to know what i mean scroll down to the tables and imagine
what (alpha-methyl)phenethylamines those benzaldehydes would give
i only translated the experimental part. i didn't know the english for
"Spaltrohrkolonne" (a type of fractionating column) so i translated it by
slit pipe column...
Attempt at the formylation of anisole with 1 and catalytical amounts of Ytterbium-tris(trifluoromethanesulfonate
1.08 g (10 mmol) Anisole, 0.73 g (10 mmol) 1 and 1.24 g (2 mmol) Yb(CF3SO3)3 in 10 ml nitromethane are refluxed for 24 h. After cooling, 20 ml water is added and the reaction stirred for 10 min at room temperature. The organic phase was separated and the aqueous phase extracted three times with 20 ml chloroform. The combined organic phases are dried over sodium sulfate. After removing the solvent on the rotavapor, the residue was analysed with GC. Only anisole could be detected.
Formylation of activated aromatics with formamide/P4O10/AlCl3 (general procedure)
To the aromatic compound, when necessary in a solvent, is added the given amount of P4O10. Under stirring and cooling in an ice bath first the aluminium chloride, then the formamide are added. Then the mixture is heated to the given temperature and stirred. After cooling the reaction is hydrolysed with ice and extracted three times with 30 ml chloroform. The organic phase is washed with sodium bicarbonate solution and water to make it neutral and dried over sodium sulfate. The chloroform is removed on the rotavapor and the residue fractionated under aspirator vacuum. Toluene, P4O10, AlCl3 and formamide are reacted with a 0.5:0.1:0.2:0.1 molar ratio for 3 h, yield: 9% 3. The reaction of anisole, P4O10, AlCl3 and formamide with a molar ratio of 0.4:0.1:0.2:0.1 (18h, 28°C) gives 12% 4. The same reaction with a molar ratio of 0.5:0.1:0.2:0.1 (2.5 h, 75°C) in nitromethane gives 4 with a yield of 15%. The same reaction in chlorobenzene with a molar ratio of 0.18:0.6:0.18:0.18 (4h, 60°C) gives 4 with 4% yield. N,N-Dimethylaniline, P4O10, AlCl3, Formamide are reacted with a molar ratio of 0.1:0.1:0.2:0.1 for 23 h at 20°C. There is no conversion.
Formylation of toluene with aluminium chloride and triformamide in toluene
To 46.0g (500 mmol) toluene is added 20.0g (150 mmol) AlCl3 under cooling in an ice bath. Then 5.1g triformamide (50 mmol) is added. The reaction is stirred for 5 h at 0°C. The mixture is hydrolysed with ice, neutralised with 5M NaOH and steam distilled. The organic phase of the distillate is separated, the aqueous phase extracted twice with diethylether. The combined organic phases are dried over sodium sulfate. The solvent is removed on the rotavapor and the residue distilled at aspirator vacuum. One obtains 4.74 g tolylaldehyde (79%) with bp 80°C / 12 Torr (Lit 8 bp. 82-84 °C / 12 Torr). The residue of the steam distillation is extracted three times with diethylether. The combined organic phases are dried over sodium sulfate, the solvent removed on the rotavapor and the residue distilled at the oil pump. One obtains 11.1 g (93%) 2a with a bp of 165°C/0.001 Torr.
Formylation of aromatic compounds with triformamide/AlCl3 (cf tables 2-4) (general procedure)
To a 2 M solution of the aromatic compound in the given solvent is added the corresponding amount of lewis acid. Under stirring at the given temperature triformamide is added, when necessary the mixture is stirred for some time, the cooling bath is removed and the reaction stirred for the given amount of time at room temperature. In order to release the aldehyde, ice water (about 200 ml, if 40 mmol of the aromatic compound are formylated) is added under agitation and the mixture heated for 20 min to 50°C.
Method A) The hydrolysed reaction is steam distilled (Note 6). The organic phase of the distillate is separated and the aqueous phase extracted three times with the given solvent (when alkylating 40 mmol, about 15 ml). After drying of the combined organic phases, the solvent is distilled off using a 30 cm vigreux column. The aldehyde is obtained by fractionating the residue through a 15 or 30 cm vigreux column.
Method B) The organic phase of the hydrolysed reaction is separated. The aqueous phase is extracted four times with the given solvent (when alkylating 40 mmol, about 20 ml). The combined organic phases are dried over sodium sulfate and the solvent removed by distilling through a 30 cm vigreux column. The aldehyde is obtained by vacuum distillation of the residue (Note 7). Very high boiling compounds which crystallise, are vacuum filtered and recrystallised.
Table 2: 40 mmol of the aromatic compound are reacted with 40 mmol aluminium chloride and 40 mmol 14 in 20-25 ml dry chlorobenzene.
Deviating from the general procedure, the following compounds were synthesised:
10-Methylantracene-9-carboxyaldehyde(30)
Method A: The aldehyde crystallising from the residue of the steam distillation is vacuum filtered off and recrystallised from Water/IPA/THF 3:5:3. By evaporation of the mother liquor more product can be obtained.
10-Formyl-phenothiazin
Method B: Reaction in chlorobenzene (3°C/0.5 h and 20°C/ 1.5 h). The extraction of the water is done with DCM. The formyl compound is isolated by distillation, then recrystallised first from ethanol, then from PE/EA (10:1). Mp 145°C, Lit 28 mp 145°C.
Table 3 and 4: Size of reaction, amount of solvent and reaction conditions are given in the table
Further exploration of the formylation agent triformamide/AlCl3 (cf table 5)
4-tert-Butylbenzaldehyde (35)
(Trials nr. 1, 1a, table 5): Using 13.4 g (100 mmol) tert-butylbenzene, 13.4 g (100 mmol) aluminium chloride and 10.1 g (100 mmol) triformamide, one obtains after 15 h of reaction (reactiontemp. -15°C ---> -1°C) and the workup given for the formylation of for o-xylol 3.4g (22%) tert-butylbenzaldehyde with bp. 58-60°C/0.2 torr, Lit 29 bp 128-129°C.
The same reaction yields 6.5g (40%) 35, when twice the amount of aluminium chloride (26.8 g (200 mmol)) is used.
4-Hexylbenzaldehyde (36) and 2,4-dihexylbenzaldehyde (37)
(Trials nr. 2-4, table 5): To 6.49 g (40 mmol) n-hexylbenzene in 20 ml 1,2-dichloroethane cooled to -15°C is added aluminium chloride (amount given in table 5). The mixture is stirred for some minutes and then 4.0 g (40 mmol) triformamide is added. In trial nr. 3 the triformamide was added directly after the aluminium chloride.
The reaction is stirred for 15-17h under moisture protection, letting the temperature slowly climb to the value given in table 5. Ice water is added carefully, after completed hydrolysis, the organic phase is separated and the aqueous phase extracted three times with 15 ml 1,2-dichloroethane. The combined organic phases are dried over sodium sulfate and the 1,2-dichloroethane is distilled off under atmospheric pressure. The aldehydes are obtained by fractionating through a 30 cm slit pipe column: 4-hexylbenzaldehyde (36) bp 87-93°C/0.1 torr, lit 30 bp 70°C/2x10-5 torr and 2,4-dihexylbenzaldehyde (37), bp 140-145°C/0.1 torr nD/20=1.5038.
C19H30O Calculated: C 83.15 H 11.02
(274.45) Found: C 82.88 H 11.07
4-Cyclohexylbenzaldehyde (38) and 2,4-dicyclohexylbenzaldehyde (39)
(Trial nr. 5, table 5): Similarly to the previous trial, one obtains from 6.41 g (40 mmol) cyclohexylbenzene in 20 ml 1,2-dichloroethane, 10.7 g (80 mmol) aluminium chloride and 4.0 g (40 mmol) triformamide 4.56 g (61%) 38 with bp 85-119°C/0.2 torr, lit 31 bp 160°C/12 torr and 1.14 g (10%) 39 with bp 140°C/0.2 torr.
C19H26O Calculated: C 84.39 H 9.69
(270.41) Found: C 84.64 H 9.71
4-Cyclohexylbenzaldehyde and 2,4-dicyclohexylbenzaldehyde (bigger preparation)
(Trial nr. 6, table 5): To 80.1 g (500 mmol) cyclohexylbenzene in 250 ml dry 1,2-dichloroethane cooled to -15°C are added under stirring 133.4 g (1000 mmol) AlCl3 during 3 min. During the addition, the temperature of the mixture increases for some degrees centigrade.
The mixture is cooled to -15°C, stirred for 8 min and 50.5g (500 mmol) triformamide is added. The reaction is stirred for 24h under moisture protection, while the temperature is slowly (!) allowed to climb to 9°C. Then the yellow-brown, viscous and nearly homogeneous mixture is poured slowly into 2.5 l ice with stirring. The reaction is stirred for some minutes, the organic phase separated and the aqueous phase extracted three times with 90 ml 1,2-dichloroethane. The combined organic phases are dried over anhydrous sodium sulfate and the solvent removed by distillation under atmospheric pressure (final temperature of the oil bath: about 200°C).
The residue, a tenacious yellow-brown oil, is fractionated at the oil pump through a silver plated 40 cm vigreux column. After a little forerun (cyclohexylbenzene) one obtains a first fraction of 31.1g (31%) 38 with bp of 80-85°C/0.1 torr. The second fraction with a bp of about 137-145°C/0.1 torr, consists mainly of 2,4-dicyclohexylbenzaldehyde. By further fractionating through a split pipe column, one obtains 11.7g (17%) 39 as colourless, viscous oil with bp about 137°C/0.1 torr.
5-Isopropyl-2-methylbenzaldehyde
(Trials nr. 7, 8, table 5): Using 13.4 g (100 mmol) p-cymene, 26.8 g (200 mmol) aluminium chloride and 10.1 g (100 mmol) triformamide in 50 ml 1,2-dichloroethane, one obtains after 16 h of reaction (reaction temperature -15°C --> -1°C) and workup as previously described 5.1 g (31%) 5-isopropyl-2-methylbenzaldehyde.
In the same way, 5.37 g (40 mmol) p-cymene, 5.35 g (40 mmol) aluminium chloride and 4.04 g (40 mmol) triformamide in 20 ml 1,2-dichloroethane give 1.51 g (28%) 5-isopropyl-2-methylbenzaldehyde with a bp of 51-52°C/0.2 torr, lit 31 bp 125°C/20 torr.
p-Phenoxybenzaldehyde
(Trials nr. 9 and 10, table 5): 10.7g (80 mmol) AlCl3 is added under the conditions given in table 5 to a mixture of 6.81 g (40 mmol) diphenylether and 20 ml 1,2-dichloroethane. Some minutes later 4.0 g (40 mmol) triformamide is added. The reaction is stirred under moisture protection (temperature and reaction time according to table 5). Then water is added carefully. To complete the hydrolysis, one heats about 20 min to 50°C. After cooling the organic phase is separated and the aqueous phase extracted twice with 20 ml 1,2-dichloroethane. The combined organic phases are dried over sodium sulfate. After evaporation of the 1,2-dichloroethane one obtains a tenacious, nearly colourless oil, the fractionating of which yields p-phenoxy-benzaldehyde (41) with bp 105-110°C/0.1 torr, Lit 22 bp 158-159°C/4 torr. Yields see table 5.
Notes
-----
6) The residue of the steam distillation contains the formamids 2. They can be extracted with hot ethanol.
7) The residue of the distillation contains the formamids 2. They can be extracted with hot ethanol.
Table 2
Head:
Reactant / Reaction conditions Temperature(°C)/Time(h) / Formula / Product Name / Yield (%) (Workup) / bp (°C/Torr)
Raumtemp. = room temperature
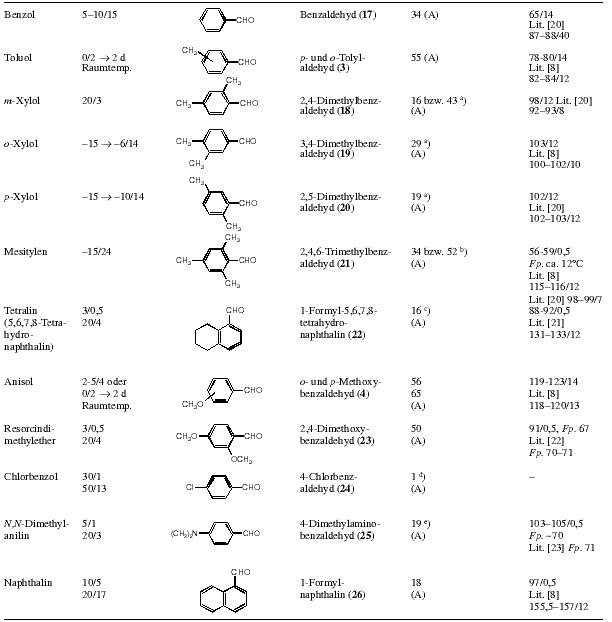
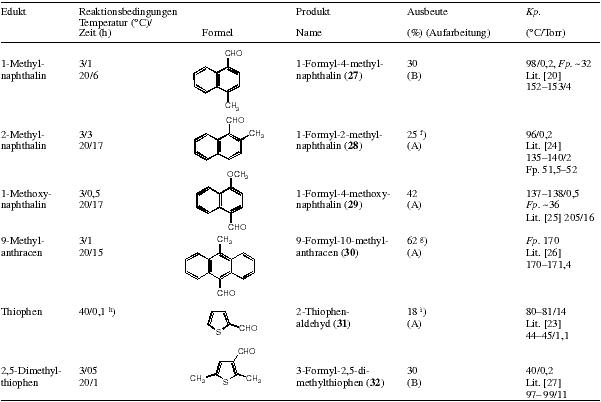
Notes:
a) Molar ratio aromatic compound/triformamide/AlCl3 = 1:1:2
b) The higher yield results from a molar ratio aromatic compound/triformamide/AlCl3 = 1:2:2
c) to a smaller extent there are cross alkylations.
d) Yield of aldehyde determined by 1H-NMR spectroscopy.
e) Small amounts of tris(4-dimethylaminophenyl)methane as secondary product.
f) The compound is contaminated by two additional isomers.
g) Molar ratio aromatic compound/triformamide/AlCl3 = 1:2:2
h) The reactants are combined at -20°C
i) Big amounts of insoluble resinous secondary products
Table 3
Head:
Trial nr. / Aromatic compound (quantity (mol)) / Triformamide (quantity (mol)) / Solvent / Activator (quantity (mol)) / Reaction temperature (°C) / Reaction time (h) / Aldehyde (Yield (%)) (Workup)
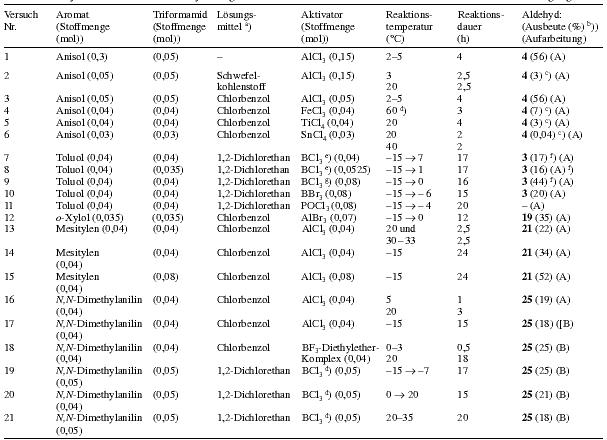
Notes:
a) Amount of solvent: 20-30 ml
b) The yields are calculated under the assumption that the aromatic compound and triformamide react in a 1:1 molar ratio.
c) Yield of aldehyde determined using 1H-NMR spectroscopy
d) The lewis acid and the formamide are added at 20°C and then the temperature is slowly augmented to the given value
e) The BCl3 was added as 1M solution in heptane
f) p:o ratio about 5:1
g) The BCl3 was added in it's condensed form
Table 4
Head:
Trial nr. / Substrate (aromatic compound) Quantity (mol) / Quantity of triformamide (mol) / Quantity of AlCl3 (mol) / Solvent / Reaction conditions Temperature(°C)/Time(h) / Aldehyde / Yield (%) (Workup)

Notes:
a) Amount of solvent: 20-25 ml
b) The corresponding N-Diarylmethyl-formamide forms as secondary product
c) 14/AlCl3 in chlorobenzene at -15°C, aromatic compound added drop by drop during 90 min.
d) Mixture of AlCl3, aromatic compound and chlorobenzene added to 14 in chlorobenzene in aliquots
Table 5
Head:
Trial nr. / Aromatic compound / Reaction conditions Temperature(°C)/Time(h) / Product(s) / Yield (%) / bp (°C/torr)
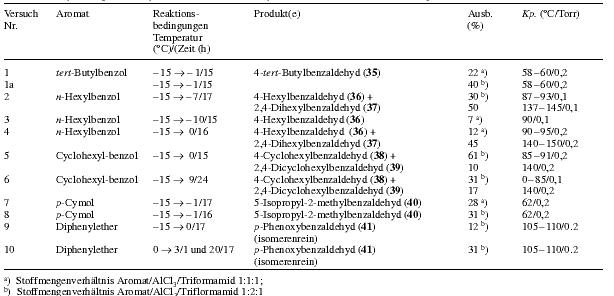
Notes:
a) Molar ratio aromatic compound/AlCl3/triformamide 1:1:1
b) Molar ratio aromatic compound/AlCl3/triformamide 1:2:1
(Distinctive Doe)
07-21-02 12:03
No 335437
Hypo
You did the hard part
Thanks
Foxy
J. Prakt. Chem. 2000, 342, No. 3
Orthoamide. LIII
Eine neue, breit anwendbare Synthese für aromatische Aldehyde
Willi Kantlehner, Markus Vettel , Alexander Gissel, Erwin Haug, Georg Ziegler, Michael Ciesielski, Oliver Scherr, Richard Haas
Abstract
Diformamide (1) reacts with activated aromatic compounds like toluene, anisole, m-xylene, 1,2-dimethoxybenzene in the presence of AlCl3 to give N-(diarylmethyl)-formamides 2a - d, the corresponding aromatic aldehydes 3 - 6 are formed as by-products in low yields. From N,N-dimethylaniline and 1/AlCl3 the triphenylmethane derivative 7 can be obtained. The reaction of anisole with N-methyl-diformamide (9) affords the formamide 10. The mixture of formamide, P4O10 and AlCl3 reveals to be a reagent which is capable to formylate toluene and anisole, resp. Triformamide (14)/AlCl3 is an effective formylating system which allows the preparation of aromatic aldehydes (e.g. 3,4,17 - 32) from the corresponding aromatic hydrocarbons. Aluminiumchloride can be replaced by borontrichloride. The yields of the formylation reactions depend strongly from the reaction conditions (molar ratio: aromatic hydrocarbon/AlCl3/14; solvent, reaction temperature). The scope of the reaction covers nearly complete those of the Gattermann-Koch-, Gattermann- and Vilsmeier - Haack-reaction.
Those who give up essential liberties for temporary safety deserve neither liberty nor safety
(Distinctive Doe)
07-21-02 12:56
No 335446
(Rated as: excellent)
New formylating agents - preparative procedures and mechanistic investigations.
Bagno, Alessandro; Kantlehner, Willi; Scherr, Oliver; Vetter, Jens; Ziegler, Georg.
European Journal of Organic Chemistry (2001), (15), 2947-2954.
Abstract
The reactivity of new formylating agents related to formamide has been investigated both experimentally and theoretically. The reaction in 1,2-dichloroethane between tris(diformylamino)methane (2) and several arenes, catalyzed by AlCl3 or BCl3, was shown to proceed in good yields to afford the corresponding para-substituted aldehydes. The nature of the active electrophilic species was also investigated theoretically. Thus, the relative stability of the O- and N-protonated forms, as well as those of AlCl3 adducts, of several formylating agents - diformamide, triformamide, N,N,N,N-tetraformylhydrazine, and tris(diformylamino)methane - were determined in the gas phase and in water or DCE by means of DFT calculations at the B3LYP/6-311++G(d,p) level, the solvents being modeled with the IPCM method. The amide oxygen atom in all cases appeared to be the most basic site, both in the Brønsted and Lewis sense, constituting a first step towards the understanding of the mechanism of this reaction.
Introduction
Despite the great importance of (hetero)aromatic aldehydes as intermediates in the chemical and pharmaceutical industries, and the continuing intense research in this field, there is still a need for new synthetic methods for the introduction of the aldehyde group into (hetero)aromatics. The introduction of a formyl group by C-C bond formation is generally achieved by means of various kinds of electrophilic aromatic substitutions, which can be subdivided further into two types of reactions: (a) reactions involving the acid-promoted generation of a formyl cation or a precursor thereof, such as formyl fluoride/boron trifluoride (Olah formylation),{1} CO/HCl (Gattermann-Koch reaction),{1,2} CO/HF,[1] HCN/HCl (Gattermann reaction),{3} and the Vilsmeier reagent,{1} formed from disubstituted formamides and phosphorus oxychloride or phosgene, and (b) reactions yielding primary products which are immediately oxidized to aldehydes, such as the synthesis of salicylaldehydes from phenols and paraformaldehyde in the presence of SnCl4/trioctylamine{1,4} or the Duff reaction, yielding aromatic aldehydes from activated aromatic compounds and urotropine in the presence of acids.{1,5}
Nowadays, the Vilsmeier (or Vilsmeier-Haack) reaction is the most common method for formylation of aromatic rings. Nevertheless, it, like the other methods, has some significant disadvantages. The formylating agents in these reactions are often toxic (as in the cases of carbon monoxide, hydrogen cyanide, formyl fluoride, and phosgene), and they are also difficult to handle in large amounts. Furthermore, the scope of these reactions is limited. The Vilsmeier-Haack reaction is the method with the widest scope, but it is incapable of formylating simple alkylarenes (unless they are much more reactive than benzene). A further difficulty is the occurrence of phosphorus compounds in wastewater, which is a serious environmental problem, and the formation of highly toxic, carcinogenic dialkylcarbamoyl chloride in a side reaction. The dichloromethyl methyl ether/Lewis acid formylating system has an even wider scope than the Vilsmeier-Haack reagent, but it has not yet found practical use.{1,3,6}
Formamide Derivatives as Formylating Agents
Some time ago, we reported on a new synthetic method for the formylation of (hetero)aromatic compounds using triformamide (1)/AlCl3 as the formylating system (Scheme 1).{7,8} This reaction is useful for the formylation of a wide range of substrates, including unsubstituted and alkyl-substituted aromatics, aromatic ethers, tertiary aromatic amines, fused aromatic rings, and thiophenes.
Scheme 1. New formylating agents based on formamide derivatives
----------------------------------------
(1) triformamide
(2) tris(diformylamino)methane
(3) tris(dichloromethyl)amine
(4) N,N,N,N-tetraformylhydrazine
----------------------------------------
In this paper we wish to introduce some other reagents based on formamide derivatives that can be used to formylate (hetero)aromatic compounds. We also present results of computational investigations aimed at elucidation of the reaction intermediates of these formylations.
The typical mechanism of electrophilic aromatic substitution reactions involves, as a preliminary step, the formation of the actual electrophilic species. In the case of amides, it is assumed that the Lewis basic site is the oxygen atom, by analogy with the behavior towards protonation.{9} However, there is no guarantee that the behavior towards the proton exactly matches that towards other Lewis acids. Thus, although one can easily formulate the generation of the electrophilic species as a Lewis acid/base process involving the amide oxygen atom as the basic site, the actual nature of the active species must be elucidated independently. For this purpose, we have carried out quantum chemical calculations aimed at quantitative determination of the extent of preference of formylating agents for protonation or Lewis acid complexation at either the oxygen or nitrogen atom in the amide group.
Formylations with Tris(diformylamino)methane
During the preparation of triformamide (1),{11,12} tris(diformylamino)methane{10} (2) is formed as a by-product.{8} In an improved method, 2 can be prepared from triformamide and sodium diformamide{13,14} in 80% yield.{8,15}
Tris(diformylamino)methane (2) is a stable, high-melting (142 °C), nonhygroscopic, and nonetching compound. The compound is nearly odorless and easy to handle. Although toxicological data are not available, its manipulation hazards may be estimated as similar to those of formamide.
Tris(diformylamino)methane (2) contains six formyl groups, plus an additional one masked as an orthoamide function, which seems to set a record for this type of compounds. In the presence of (strong) Lewis acids, it is capable of formylating activated arenes.{8,15} The efficiency of 2 as a formylating agent is higher than that of triformamide (1), since up to three out of the seven formyl groups in 2 can be used synthetically, whereas 1 can transfer only one of its three formyl groups.
In the presence of AlCl3 or BCl3, alkylarenes and aromatic ethers are formylated by tris(diformylamino)methane (2) in moderate to good yields (see Table 1). With AlCl3 as the Lewis acid, yields are highest if a tris(diformylamino)methane/AlCl3 ratio of 1:6 to 1:8 is used (see Table 2). This corresponds to a ratio of 1:2 with regard to the number of formyl groups transferred, a value coincident with the ratio found in the Friedel-Crafts acylation of esters and anhydrides. 1,2-Dichloroethane proves to be the best solvent for the reaction, although other solvents, such as chlorobenzene and carbon disulfide, are also suitable. Solvents containing nitro groups, such as nitromethane and nitrobenzene, seem to suppress the formylating reaction, probably because of complexation of the Lewis acid.
Table 1. Formylation of activated aromatic compounds with 2/Lewis acid in 1,2-dichloroethane
----------------------------------------
Substrate
Lewis acid(a)
Reaction conditions(b)
Procedure/workup(b)
Yield [%](c)
b.p. [°C/Torr] Ref.
----------------------------------------
Toluene
AlCl3
-150 (20)
I
55
84/12
204-205 °C/760 Torr {26}
----------------------------------------
Cumene
AlCl3
-13-1 (14)
II/A
38
41-42/0.2(d)
103-104 °C/10 Torr {26}
----------------------------------------
tert-Butylbenzene
AlCl3
-15-1 (16)
II/B
33
63/0.2
128-129 °C/760 Torr {27}
----------------------------------------
Hexylbenzene
AlCl3
-13-120 (15/2)
II/B
55
90-93/0.2
70 °C/2·10-5 Torr {28}
----------------------------------------
o-Xylene
AlCl3
-19-1 (15)
I
42(e)
96/0.2
223-225 °C/760 Torr {26}
----------------------------------------
p-Cymene
AlCl3
-15-3 (15)
II/B
45(f)
50-51/0.2
125 °C/20 Torr {29}
----------------------------------------
Resorcinol dimethyl ether
AlCl3
-132 (15)
II/B
45(g)
93/0.2 (m.p. 68 °C)
165 °C/10 Torr {26} (m.p. 70-71 °C {31})
----------------------------------------
p-Cymene
BCl3
-13-1 (15)
I
18(f)
50-51/0.2
125 °C/20 Torr {29}
----------------------------------------
Anisole
BCl3(h)
-101 (14)
II/B
20
54/0.02
118-120 °C/13 Torr {5}
----------------------------------------
(a) Molar ratio substrate/2/Lewis acid (3:1:6).
(b) Initial and final reaction temperature [°C], and reaction time [h] in parentheses. See Exp. Sect. for synthetic and workup procedures.
(c) Product is always the p-substituted benzaldehyde. Yields are based on tris(diformylamino)methane under the assumption that 1 mol of the reagent supplies 3 mol of formyl groups.
(d) nD20=1.5390 (1.5301[26]).
(e) Product: 3,4-dimethylbenzaldehyde.
(f) Product: 5-isopropyl-2-methylbenzaldehyde.
(g) Product: 2,4-dimethoxybenzaldehyde.
(h) Molar ratio substrate/2/Lewis acid (3:1:4).
Table 2. Influence of molar ratio of reagents on yield of formylation of o-xylene with tris(diformylamino)methane (2) in the presence of AlCl3 (Procedure I)
----------------------------------------
Molar ratio(a)<o-Xylene -- AlCl3> Yield%(b)
----------------------------------------
<3 --- 4(c)> 42%
----------------------------------------
<3 --- 5> 46%
----------------------------------------
<3 --- 6(c)> 42%
----------------------------------------
<4 --- 5(c)> 47%
----------------------------------------
<4 --- 6> 59%
----------------------------------------
<4 --- 8> 65%
----------------------------------------
<6 --- 6(c)> 44%
----------------------------------------
<6 --- 6> 63%
----------------------------------------
<6 --- 8> 72%
----------------------------------------
<6 --- 10> 75%
----------------------------------------
<6 --- 12> 64%(d)
----------------------------------------
<14(e) --- 12> 65%
----------------------------------------
<15.3(e) --- 4> 45%
----------------------------------------
(a) With respect to 2.
(b) Product is 3,4-dimethylbenzaldehyde. Yield calculated for transfer of 3 formyl groups per molecule 2.
(c) Aged AlCl3 (partially hydrolyzed by repeated opening of the container).
(d) 1,2-Bis(3,4-dimethyphenyl)ethane (3 %) was obtained as a by-product.
(e) Reaction performed without additional solvent.
The reaction features a high para selectivity in the substrate, consistent with the high steric bulk of the attacking species. As can be appreciated from Table 1 and Table 2, in order to achieve satisfactory yields with formamide derivatives bearing one or more diformylamino groups, it is necessary to use at least 2 equiv. of Lewis acid. It is hence very likely that the primary adduct reacts with further Lewis acid to form the formylating species.
Lastly, we emphasize that the method we have presented can be performed using only stoichiometric amounts of the formamide derivative, and 2 equiv. of Lewis acid per formyl group transferred. E-factors (kg of waste per kg of product) of the new formylation procedures are in the range from 4 to 11; further investigations will show whether these values can be reduced by use of other types of catalysts. The comparable method with dichloromethyl methyl ether often employs a large excess of the formylating mixture (up to 18 equiv.),{16,17} and the regioselectivity is rather low.{18}
Formylation with Other Formamide Derivatives
The perchlorinated derivative of triformamide, tris(dichloromethyl)amine (3),{12} can be prepared from N,N-dimethylformamide and phosgene, followed by photochlorination of the resulting N,N-dimethylformamide chloride. In the presence of various Lewis and Brønsted acids, 3 also proves to be a good formylating agent, and it is suitable for the formylation of a wide range of aromatic compounds.{8} Thus, toluene reacts with 3/AlCl3 to give tolualdehyde, in a yield of 70% and a p/o ratio of 30:1. Similarly, anisaldehyde can be prepared from anisole and 3/ZnCl2 in 62% yield (p/o 24:1) (see Exp. Sect.). However, this complex subject will be dealt with in more detail in a forthcoming paper. Preliminary studies on the formylating ability of another formamide derivative, N,N,N,N-tetraformylhydrazine (4), are also encouraging.{19,20}
**********
Skiped Mechanism Stuff
**********
Conclusion
Arene formylation can conveniently be carried out in satisfactory yields with formamide derivatives in the presence of Lewis acids. Quantum chemical calculations provide cogent indications of the nature of the electrophilic species involved in this reaction. Thus, in the attack by a proton or by AlCl3 (protonation or Lewis acid adduct formation, respectively), the amide oxygen atom is the most basic site, both in the gas phase and in water or DCE as solvents. The formation of a chelate complex between the two adjacent carbonyl oxygen atoms in a diformamide group and AlCl3 is also supported. All these results reinforce the proposition that the attacking electrophilic species is formed by attachment of the Lewis acid to the oxygen atom of the formamide derivatives, and endorse the proposed reaction mechanism (Scheme 5).
Experimental
Formylation of Toluene with 2 and Various Amounts of AlCl3. - (Typical) Procedure I: Dry AlCl3 (90 mmol) was added with stirring to a mixture of toluene (45 mmol) and dry 1,2-dichloroethane (25 mL), cooled in an ice/salt bath. After a few minutes, tris(diformylamino)methane (2) (15 mmol) was added. The reaction mixture was stirred in the cooling bath with exclusion of moisture for 20 h, during which the temperature rose to 0 °C. The viscous, reddish brown mixture was carefully hydrolyzed by addition of 100 mL of ice-cold water, and steam-distilled. The organic layer of the distillate was separated, and the aqueous phase extracted three times with 10 mL of 1,2-dichloroethane. The combined organic layers were dried with sodium sulfate and filtered, and the solvent was evaporated at ordinary pressure. p-Tolualdehyde (b.p. 84 °C/12 Torr) (204-205 °C/760 Torr[26]) was isolated by fractional vacuum distillation. Yield 25 mmol (55%).
Formylation of Aromatic Compounds with 2/Lewis Acid. - (General) Procedure II: A stirred mixture of the aromatic compound (40-60 mmol) and 1,2-dichloroethane (25-40 mL) was cooled in an ice/salt bath. The Lewis acid was added first, over 5 min, followed by tris(diformylamino)methane (2). During the reaction time stated, the temperature of the reaction mixture rose from ca. -15 °C to 0 °C (see Table 1). After hydrolysis by careful addition of 100 mL of water, the organic layer was separated and the aqueous phase was extracted three times with 10 mL of 1,2-dichloroethane. - Workup A: The organic layers were combined, and the solvent was evaporated at reduced pressure. The residue was treated with 100 mL of a saturated sodium hydrogen sulfite solution, 3 mL of methanol, and ca. 250 mg of tetrabutylammonium hydrogen sulfate. The bisulfite adduct separated upon stirring and was isolated by filtration. The adduct was cleaved by addition of either 100 mL of 8% NaHCO3 solution or 20 mL of 10% HCl. The aldehyde was extracted from the mixture three times with 10 mL of ether. The combined organic layers were dried with sodium sulfate and filtered, and the solvent was evaporated. The aldehyde was obtained by fractional distillation of the residue through a 15-cm Vigreux column. - Workup B: The combined organic layers were dried with sodium sulfate and filtered, and the solvent was evaporated at reduced pressure. The aldehyde was obtained by fractional distillation of the residue through a 10-cm Vigreux column.
Formylation of Aromatic Compounds with 3/Lewis Acid. - (a) Tolualdehyde from 3 and AlCl3: Compound 3 (7.3 g, 30 mmol) was added over 10 min, at 5 °C and with stirring, to a cooled mixture of dry toluene (25 g, 0.27 mmol) and AlCl3 (11 g, 80 mmol). The cooling bath was removed and the mixture was stirred for 15 h at 20 °C. The reaction mixture was hydrolyzed with 50 mL of water and steam-distilled. The organic phase of the distillate was separated and the aqueous phase was extracted three times with CH2Cl2. The organic phases were combined and dried with sodium sulfate. The drying agent was removed by filtration. The solvents were removed from the filtrate by distillation at ordinary pressure. Tolualdehyde (2.31 g, 70%; 97% p-isomer, 3% o-isomer) was isolated by distillation (b.p. 80 °C/12 Torr). - (b) Anisaldehyde from 3 and ZnCl2: ZnCl2 (2.18 g, 31.6 mmol) was added to a solution of 3 (4.2 g, 15.8 mmol) in anisole (20 mL, 187 mmol), whereupon the mixture turned red. The mixture was heated with stirring for 3 h at 60-65 °C, hydrolyzed with 100 mL of water, and steam-distilled. Dichloromethane (30 mL) was added to the distillate in order to produce better phase separation. The organic phase was separated and the aqueous phase was extracted three times with 50 mL of dichloromethane. The combined organic phases were dried with sodium sulfate. The solvents were evaporated, and the residue was distilled in vacuo through a 20-cm Vigreux column. Yield: 1.33 g (62%) anisaldehyde (96% p-isomer, 4% o-isomer), b.p. 80 °C/0.01 Torr.
References
{1} G. Simchen, Methoden Org. Chem. (Houben-Weyl), 1983, vol. E3.
{2} E. Winterfeldt, Methoden Org. Chem. (Houben-Weyl), 1983, vol. E3.
{3} G. A. Olah, S. J. Kuhn, Friedel-Crafts and Related Reactions (Ed.: G. A. Olah), Interscience Publ., New York, 1964, vol. III/2.
{4} G. Casiraghi, G. Casnati, G. Puglica, G. Sartori, G. Terenghi, J. Chem. Soc., Perkin Trans. 1 1980, 1862
{5} J.C. Duff, J. Chem. Soc. 1941, 574
{6} A. Rieche, H. Groß, E. Höft, Chem. Ber. 1960, 93, 88
{7} W. Kantlehner , M. Vettel, A. Gissel, E. Haug, G. Ziegler, M. Ciesielski, O. Scherr, R. Haas, J. Prakt. Chem. 2000, 342, 297
{8} W. Kantlehner, E. Haug, G. Ziegler, O. Scherr, M. Ciesielski: Neue, umweltfreundliche, gewerbetoxikologisch unbedenkliche Aldehydsynthesen (Varianten der Vilsmeier-Haack-Reaktion), Final Report to BMBF Project (Förderkennzeichen 01 Z 9502), 1998.
{9} A. Bagno , G. Scorrano , J. Phys. Chem. 1996, 100, 1536
{10} Crystal structure: W. Frey, W. Kantlehner, G. Ziegler, O. Scherr, Z. Kristallogr. 2001, 216, 97
{11} E. Allenstein, V. Beyl, W. Eitel, Chem. Ber. 1969, 102, 4089
{12} K. Grohe, E. Klauke, H. Holtschmidt, H. Heitzer, Justus Liebigs Ann. Chem. 1969, 730, 140
{13} E. Allenstein, V. Beyl, Chem. Ber. 1967, 100, 3551
{14} H. Yinglin, H. Hongwen, Synthesis 1990, 122.
{15} W. Kantlehner, G. Ziegler, M. Ciesielski, O. Scherr, M. Vettel , Z. Naturforsch., Teil B 2001, 56 , 105
{16} A. Arduini, G. Manfredi, A. Pochini, A.R. Sicuri, R. Ungaro, J. Chem. Soc., Chem. Commun. 1991, 936
{17} T. Shimizu, S. Hiranuma, T. Watanabe, M. Kirihara, Heterocycles 1994, 38, 243
{18} G.A. Olah, L. Ohannesian, M. Arvanaghi, Chem. Rev. 1987, 87, 671
{19} W. Eitel, Dissertation Universität Stuttgart 1971, p. 42.
{20} Proceedings of the 4th Iminium Salt Conference, Rechenberg/Stimpfach, September 14-16, 1999.
{21} M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, V.G. Zakrzewski, J. A. Montgomery, Jr., R.E. Stratmann, J.C. Burant, S. Dapprich, J.M. Millam, A.D. Daniels, K.N. Kudin, M.C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G.A. Petersson, P.Y. Ayala, Q. Cui, K. Morokuma, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J. Cioslowski, J.V. Ortiz, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, J.L. Andres, C. Gonzalez, M. Head-Gordon, E.S. Replogle and J.A. Pople,
Gaussian 98, Revision A.7, Gaussian, Inc., Pittsburgh PA, 1998.
{22} J.B. Foresman, T.A. Keith, K.B. Wiberg, J. Snoonian, M. J. Frisch, J. Phys. Chem. 1996, 100, 16098
{23} A.E. Reed, L.A. Curtiss, F. Weinhold , Chem. Rev. 1988, 88, 899
{24} A. Bagno, B. Bujnicki, S. Bertrand, C. Comuzzi, F. Dorigo, P. Janvier, G. Scorrano, Chem. Eur. J. 1999, 5, 523.
{25} F.B. van Duijneveldt , J.G.C.M. van Duijneveldt-van de Rijdt, J.H. van Lenthe, Chem. Rev. 1994, 94, 1873
{26} R.C. Weast (Ed.), Handbook of Chemistry and Physics, 60th ed., CRC Press, Boca Raton (Florida), 1979-1980.
{27} G.Y. Han, P.F. Han, J. Perkins, H.C. McBay, J. Org. Chem. 1981, 46, 4695
{28} J. Frahn, A.D. Schlueter, Synthesis 1997, 1301.
{29} C.T. Lester, R.E. Donaldson, J.C. Oswald , J. Am. Chem. Soc. 1949, 71, 1502.
{30} L. Rousset , Bull. Soc. Chim. Fr. 1897, 17, 810.
{31} A. Kreutzberger , Arch. Pharm. Ber. Dtsch. Pharm. Ges. 1969 , 302 , 828.
{32} E. P. Hunter, S. G. Lias, J. Phys. Chem. Ref. Data 1998 , 27 , 413 . Available on the Internet as http://webbook.nist.gov
(Official Hive Translator)
07-21-02 18:23
No 335491
This is absolutely cool, as far as the chemistry goes!
And the widest range of applicability makes this route truely unique.
However, WTF is triformamide? I tried to find a synth of this compd and the word gave nothing on espacenet and 1 single hit on USPatent!
Looks like it's pretty obscure. Anyone has any info on how it's made or something?
Antoncho
(Official Hive Approximator)
07-21-02 19:14
No 335500
it's triformylamine (N with three CHO- groups).
in the paper they say they used the preparation given in:
E.Allenstein, V.Beyel, Chem. Ber. 1969, 102, 4089
(Hive Bee)
07-21-02 20:59
No 335516
Triformylamine used on o-xylene gives 3,4-dimethylbenzaldehyde. anybody up for making some 3,4-dimethyl(meth)amphetamine
(Official Hive Translator)
07-22-02 03:05
No 335591
- just someone needs first to get that triformamide reference from Hypo's previous post.
Wonder what can bee used to make such a weird compound....
Antoncho
(Chief Bee)
07-22-02 04:51
No 335619
Isn't the common buffer TRIS<tm> made of tris-(hydroxymethyl)-amine? Perhaps that can be selectively oxidized to triformylamine?
(Distinctive Doe)
07-22-02 06:14
No 335638
Ya had me excited for a second. Considering the mood I'm in thats quite amazing.
TRIS is this 2-Amino-2-(hydroxymethyl)-1,3-propanedio
Those who give up essential liberties for temporary safety deserve neither liberty nor safety
(Official Hive Approximator)
07-22-02 15:17
No 335771
(Rated as: good read)
ok, i fetched Chem. Ber. 102, 4089-4103 (1969) and
Liebigs Ann. Chem. 730, 151-157 (1969) (interesting case
of simultaneous invention, huh??), both in german.
i fear this ain't exactly kitchen chemistry
let's hope that the renewed interest in these alkylation compounds
leads to commercial products or easier preparations!
otoh, we have some crazy bees, so here are the relevant parts:
Liebigs Ann. Chem. 730, 151-157 (1969):
DMF is reacted with phosgene and chlorine to give tris-dichloromethyl-amine, which is reacted with anhydrous formic acid to give triformamide.
Tris-dichloromethyl-amine (3h). - Phosgene is introduced into a solution of 146 g (2 mol) DMF in 400 ccm chloroform for 4-5 hours at 20-30°. The reaction is then chlorinated with UV light for 12 h at 40-60° and for 30 h at 80-100°. After recrystallisation of the colourless mush from tetrachloromethane or benzine, one obtains about 300 g (57%) 3h with mp 171-173°.
[...]
Triformamide (8b). - 79.5 g (0.3 mol) 3h is mixed with 120 ccm anhydrous formic acid. The reaction is stirred for 2-3 h at 20-30° then heated for 4-5 h to 90-100°. The excess formic acid is removed under vacuum and the colourless residue is kept in a dissicator over KOH. Purification is done by resublimating at 70-80° (water bath)/0.05 torr. Colourless crystals with mp 92-04°; Yield 22g (73%).
what they don't say in the preparation of triformamide, but in the preparation of n-dichloromethyl-diformamide is that there is "roaring" HCl formation when mixing formic acid with 3h.
Chem. Ber. 102, 4089-4103 (1969):
reacting equimolar amounts of n,n-diformyl-acetamide with diformamide gives triformamide.
n,n-diformyl-acetamide (which according to the authors is a good formylating agent by itself!) can be obtained without interfering secondary products by reacting silver diformamide (obtainable by reacting silver nitrate with sodium diformamide in aqueous solution) and acetyl chloride in 50% yield or with secondary products by directly reacting sodium diformamide with acetyl chloride with 30-40% yield. the latter reaction produces triformamide which can not be separated from the n,n-diformyl-acetamide. but if it is used to make triformamide anyway, this might not be a big problem!
4. Reaction of sodium diformamide with acetyl chloride: Under moisture protection a mixture of 102.7 g (1.081 mol) 5 (=sodium diformamide) and 101.8g (1.297 mol) acetyl chloride in 200 ccm absolute ether is refluxed with stirring for 20 h. The precipitate is filtered off and extracted for 12 h in a soxhlet with 500 ccm absolute ether. Without thorough extraction, the yield of the ether soluble compounds is diminished considerably. The etheric filtrate and the etheric extract are combined, the ether and the excess acetyl chloride stripped under aspirator vacuum at 10-15°, giving a yellowish liquid, which is vacuum fractionated through a spinning band column with 24 theoretical plates to give the following fractions: 1. bp 32-35°/10 torr, 12.9g; 2. bp 66.5°/10 torr, 57.4 g; 3. bp 114°/10 torr, yield not determined.
here is some analytical stuff, the short story is:
fraction 1: colourless liquid, acetic anhydride
fraction 2: colourless liquid, mainly diformyl-acetamide with 1.8g triformamide
fraction 3: colourless crystals (which crystallised in the top of the column -> the reason for no given yield), n-formyl-acetamide.
7. Silver diformamide: At room temperature, a freshly prepared and heavily stirred solution of 19.62g (0.2065 mol) 5 in 1.8l water is rapidly added to a solution of 30.31 g (0.1784 mol) silver nitrate in 120 ccm water. There is immediately formation of a colourless and very voluminous precipitate. After cooling to 0°, the precipitate is filtered off under cooling and washed on the buechner with 200 ccm ice cold water and 400 ccm methanol, then it is washed with stirring with a mixture of 200 ccm methanol and 200 ccm ether, filtered and washed thrice on the buechner with 100 ccm absolute ether. On drying with a mercury-diffusion pump, the colourless precipitate turns grey-violet. Yield 29.3g (91%)
[...]
8. N,N-Diformyl-acetamide (3): under moisture protection, to an ice cold and stirred suspension of 30.48g (0.1694 mol) silver diformamide in 30 ccm absolute ether is slowly added drop by drop a solution of 44.1 g (0.562 mol) acetyl chloride in 40 ccm absolute ether, and the resulting mixture is stirred for 48 h at 0°, whereupon the grey-violet silver salt is transformed into a colourless precipitate. After filtering and washing the precipitate with 100 ccm absolute ether, the combined organic phases are stripped from solvent and the liquid residue is fractionated through a spinning band column, giving the following fractions: 1. bp 30-35°/10 torr, 0.99 g; 2. bp 66.5°, 9.62 g. There was 4.11 g of a yellow brown residue, which was not further analysed.
again some analytical data: 1. is acetic anhydride, 2. is diformyl-acetamide without triformamide contamination.
13. Triformamide (4): Under moisture protection, a mixture of 22.37 g (0.3062 mol) diformamide (6) and 33.25 g (0.3063 mol) is heated at first for 6 h, then twice for 2 h to 80°. After each heating period, the liquid reaction is slowly cooled on the oil bath, giving hexagonal prisms with a length of some cm, which are isolated by decanting the liquid, then washed with little absolute ether and dried under vacuum. Analysis and IR-spectroscopy showed that the crystals were the same as 4 isolated in trial 4. Yield 18.22 g (58.9%), mp 98-99°. [...]
The liquid residue is heated in a flask fitted with a vertical water cooled liebig condenser to 100°, whereby further crystals of slightly impure 4 resublimate in the condenser. After washing with absolute ether and drying under vacuum, they showed the same analytical composition as the previous described crystal fraction, but a mp of 95-97°. Yield 4.90 g (15.8%). The yellow residue crystallised on cooling, and showed (apart from some small absorptions due to contamination) the IR-spectrum of N-formyl-acetamide.