(Chief Bee)
10-04-02 12:21
No 364157
(Rated as: excellent)
Facile preparation of N-methyl secondary amines by titanium(IV)isopropoxide-mediated reductive amination of carbonyl compounds
JCS Perkin Trans 1, 2527-31 (1998)
A simple, mild and efficient procedure for obtaining N-methyl secondary amines from aldehydes and ketones is reported. Treatment of carbonyl compounds with methylamine hydrochloride, triethylamine and titanium(IV) isopropoxide, followed by in situ sodium borohydride reduction and straightforward aqueous work-up, affords clean products in good to excellent yields.
Reductive amination of MDP2P with MeNH2.HCl, Ti(iPrO)4, NaBH4 and Et3N
A mixture of 3,4-Methylenedioxyphenyl-2-propanone (1.78g, 10 mmol), titanium(IV)isopropoxide (5.9ml, 20 mmol), methylamine hydrochloride (1.35 g, 20 mmol) and triethylamine (2.79ml, 20 mmol) in 15ml absolute ethanol (or methanol) was stirred (preferably under inert atmosphere) room temp for 810 h. Sodium borohydride (0.57g, 15 mmol) was then added and the resulting mixture was stirred for an additional 78 h at ambient temperature. The reaction was then quenched by pouring into 30ml 2M aqueous ammonia, the resulting inorganic precipitate was filtered off, and washed with 50ml dichloromethane. The organic layer was separated and the remaining aqueous layer was extracted once with 50ml dichloromethane. The combined organic extracts were next extracted once with hydrochloric acid (1 M, 25ml) to separate the neutral materials. The acidic aqueous extracts were washed once with 50ml dichloromethane, then treated with dilute aqueous sodium hydroxide to pH 1012, and extracted with 3x50ml dichloromethane. The combined organic extracts were washed with 50ml brine, dried over MgSO4 and concentrated in vacuo to afford N-Methyl-3,4-Methylenedioxyphenyl-2-amin
(Hive Bee)
10-04-02 12:47
No 364164
What Ti(iPrO)4 did they use? When I do a quick search, there is a difference between extra pure vacuum distilled (99.99%) and "normal" Ti(iPrO)4. The difference can be important, since the price difference is ca 100-fold.
There are some other articles on your site (../rhodium /redamin
WOMAN.ZIP: Great Shareware, but be careful of viruses...
(Hive Bee)
10-04-02 14:58
No 364191
Regular Ti(iPrO)4 works just fine. With this system one can get quite good yields of primary amines as well. The dry enviroment makes ammonia keen to form the imine. But as for simplicity and yields, I´d say Sunlights ketone/Pd/amine/formate is hard to beat.
Catalytic hydrogenation freak
(Hive Bee)
01-29-04 21:16
No 485344
(Rated as: excellent)
synthesis of primary and symmetrical secondary amines
Bruhaspathy Miriyala,a Sukanta Bhattacharyyab,* and John S. Williamsona
Tetrahedron 60 (2004) 14631471 (http://lego.chemistry.tripod.com/Journa
DOI:10.1016/j.tet.2003.12.024
Abstract: An efficient, general procedure for highly chemoselective reductive mono-alkylation of ammonia with ketones is reported.
Treatment of ketones with ammonia in ethanol and titanium(IV) isopropoxide, followed by in situ sodium borohydride reduction, and a
straightforward workup afforded primary amines in good to excellent yields. Reductive alkylation of ammonia with aldehydes, on the other
hand, afforded the corresponding symmetrical secondary amines selectively.
[...]
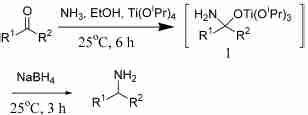
Scheme 1. Primary amines from ketones
[...]
Table 1. Synthesis of primary amines from ketones
| Entry | Starting ketone | Product primary amine | Yield (%) | Purity (%)a |
| 1 | Benzophenone | 1-Phenylethylamine | 88 | 98 |
| 2 | 4-Methoxybenzophenone | 1-(4-Methoxyphenyl)-ethylamine | 91 | 97 |
| 3 | 4-Fluorobenzophenone | 1-(4-Fluorophenyl)-ethylamine | 65 | 99 |
| 4 | 4-Trifluorobenzophenone | 1-(4-Trifluorophenyl)-ethylamine | 83 | 99 |
| 5 | 4-Trifluoromethoxybenzophenone | 1-(4-Trifluoromethoxyphenyl)-ethylamine | 83 | 97 |
| 6 | Propiophenone | 1-Phenylpropylamine | 89 | 99 |
| 7 | 4-Phenyl-2-butanone | 2-Amino-4-phenylbutane | 88 | 98 |
| 8 | 4-phenylbut-3-en-2-one | 1-Methyl-3-phenylprop-2-enylamine | 89 | 98 |
| 9 | 2,4-Dimethoxyphenylacetone 2,4-DiMeO-P2P |
2,4-Dimethoxyamphetamine | 87 | 97 |
| 10 | 4-Fluorophenylacetone 4-F-P2P |
4-Fluoramphetamine | 78 | 99 |
| 11 | Benzophenone | 1,1-diphenylmethanamine | 78 | 98 |
| 12 | 1-benzoylpiperidin-4-one | 1-benzoylpiperidin-4-amine | 96 | 100 |
| 13 | ethyl 4-oxopiperidine-1-carboxylate | ethyl 4-aminopiperidine-1-carboxylate | 93 | 100 |
| 14 | 1-BOC-4-oxopiperidine | 1-BOC-4-aminopiperidine | 88 | 98 |
| 15 | 1,4-dioxaspiro[4.5]decan-8-one 1-Ethylenacetal-1,4-dicyclohexandion |
Amino-4-cyclohexanoneethylenacetal | 91 | 99 |
| 16 | Cyclohexylphenylketone | 1-cyclohexyl-1-phenylmethanamine | 72 | 98 |
| 17 | Cyclopentanone | Cyclopropylamine | 90 | 99 |
| 18 | Cycloheptanone | Cycloheptylamine | 88 | 98 |
| 19 | 2-Adamantanone | 1-Aminoadamantan | 93 | 100 |
| 20 | Diisopropylketon | 1-Isopropyl-2-methylpropylamine | 85 | 100 |
a Purity was determined by GC or LC-MS
[...]

Scheme 2. Symmetrical secondary amines from aldehydes
Table 2. Synthesis of symmetrical secondary amines from aldehydes
| Entry | Starting aldehyde | Product secondary amine | Yield (%) | Purity (%)a |
| 21 | Benzaldehyde | Dibenzylamine | 76 | 99 |
| 22 | 2-Methylbenzaldehyde | Di-(2-methylbenzyl)-amine | 70 | 99 |
| 23 | 3-Methylbenzaldehyde | Di-(3-methylbenzyl)-amine | 71 | 99 |
| 24 | 4-Methylbenzaldehyde Toluylaldehyde |
Di-(4-methylbenzyl)-amine | 78 | 98 |
| 25 | 4-Methoxybenzaldehyde Anisaldehyde |
Di-(4-methoxbenzyl)-amine | 50 | 98 |
| 26 | 4-Fluorbenzaldehyde | Di-(4-fluorobenzyl)-amine | 75 | 98 |
| 27 | 4-Trifluorobenzaldehyde | Di-(4-trifluorobenzyl)-amine | 70 | 100 |
| 28 | 4-Dimethylaminobenzaldehyde | Di-(4-dimethylaminobenzyl)-amine | 62 | 100 |
| 29 | 3,4-Methylendioxybenzaldehyde Piperonal |
Di-(3,4-methylendioxybenzyl)-amine | 77 | 97 |
| 30 | 3-Benzyloxybenzaldehyde | Di-(3-benzyloxybenzyl)-amine | 67 | 97 |
| 31 | Indole-3-carbaldehyde | Di((1H-indol-3-ylmethyl)-amine | 65 | 98 |
| 32 | 2-Methylphenylacetaldehyde | Di-(2-phenylpropyl)-amine | 58 | 98 |
| 33 | 4-Phenylbutyraldehyde | Di-(4-phenylbutyl)-amine | 75 | 100 |
| 34 | Cyclohexanonecarbaldehyde | Di-(cyclohexylmethyl)amine | 68 | 98 |
a Purity was determined by GC or LC-MS
[...]
4. Experimental
The starting aldehydes and ketones, reagents and solvents were used as obtained from their respective suppliers without further purification. Two molar solutions of ammonia in ethanol are commercially available and were obtained from Aldrich Chemical Company, USA. IR spectra were recorded with CHCl3 as solvent (Bruker Vector 33 FTIR). 1H NMR spectra were run in CDCl3 at 400 MHz on a Bruker AM 400 spectrometer. Chemical shifts are reported in ppm referenced to TMS. Liquid chromatography and electrospray ionization mass spectrometry (EIMS) were performed with a Waters 2690 Separation Module HPLC system and a Waters/Micromass ZQ 2000 mass spectrometer in the positive ion detection mode. Components were resolved using a Waters Symmetry C18 5 mm HPLC column (2.1 x 50 mm). Flash chromatography was performed on silica gel (200400 mesh, Natland). Analytical TLC was performed on pre-coated silica gel plates with fluorescent indicators using purified solvents, followed by iodine visualization, as necessary. All products were characterized by their 1H NMR, IR and mass spectral data; identities of known compounds were established by comparison of their NMR spectral data with the values reported in the literature. The purities of the product primary and secondary amines were determined by using GC or LCMS analysis.
4.1. General procedure for the synthesis of primary amines from ketones
A mixture of the ketone (10 mmol), titanium(IV) isopropoxide (6.0 mL, 20 mmol) and ammonia in ethyl alcohol (2 M, 25 mL, 50 mmol) was stirred under argon in a capped flask at ambient temperature for 6 h. Sodium borohydride (0.6 g, 15 mmol) was then added and the resulting mixture was stirred at room temperature for an additional 3 h. The reaction was then quenched by pouring into ammonium hydroxide (2 M, 25 mL), the resulting inorganic precipitate was filtered off, and washed with ethyl acetate (25 mL x 2). The organic layer was separated and the remaining aqueous layer was extracted with ethyl acetate (25 mL x 2).
The combined organic solution was next extracted with hydrochloric acid (1 M, 30 mL) to separate the neutral materials. The acidic aqueous extracts were washed with ethyl acetate (50 mL), then treated with aqueous sodium hydroxide (2 M) to pH 1012, and extracted with ethyl acetate (50 mL x 3). The combined organic extracts were washed with brine (50 mL), dried (Na2SO4), and concentrated in vacuo to afford the corresponding primary amine.
[...]
4.2. General procedure for the synthesis of symmetrical secondary amines from aldehydes
A slurry of the aldehyde (10 mmol), titanium(IV) isopropoxide (6.0 mL, 20 mmol), ammonium chloride (1.1 g, 20 mmol) and triethylamine (2.8 mL, 20 mmol) in absolute ethanol (20 mL) was stirred under argon in a capped flask at ambient temperature for 6 h. Sodium borohydride (0.6 g, 15 mmol) was then added and the resulting mixture was stirred at room temperature for an additional 3 h. The reaction was then quenched by pouring into ammonium hydroxide (2 M, 25 mL), the resulting inorganic precipitate was filtered off, and washed with ethyl acetate (25 mL x 2).
The organic layer was separated and the remaining aqueous layer was extracted with ethyl acetate (25 mL x 2). The combined organic extracts were dried over Na2SO4, concentrated in vacuo and purified over silica gel by flash chromatography using hexanes/ethyl acetate to afford the corresponding symmetrical secondary amines.
[...]
The tendency is to push it as far as you can