10-21-02 23:22
No 370993
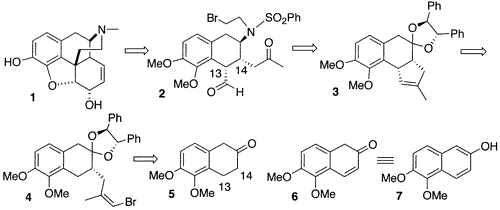
The route to morphine
In a 23-step synthetic route from 5,6-dimethoxy-â-tetralone the synthesis of (–)-morphine is presented. This strategy leads the way for the production of a number C-10, C-15 and C-16 substituted morphine analogues previously not possible.
An essential step in the synthesis was the bis-intramolecular cyclization of a keto aldehyde that afforded a tetracycle enone just 7 steps away from the final product. This enone was reduced to a single alcohol that was cyclised (on exposure to BBr3) to the pentacyclic morphine skeleton.
Removal of the phenylsulfonyl protecting group followed by reprotection produced a carbamate that was epoxidised. Regioselective ring opening of the subsequent epoxide yielded a selenide that upon oxidation and elimination produced an allylic alcohol.
Codeine was derived from the allylic alcohol and O-demethylation of codeine resulted in formation of the final product, morphine. Characterisation by thin layer chromatography and both 1H and 13C NMR provided results identical to those for the natural material.
Synthesis of (–)-morphine.
Douglass F. Taber, Timothy D. Neubert & Arnold L. Rheingold
Journal of the American Chemical Society 2002, 124(42): 12416–12417.
This is something I ran across in my email, anyone have an ACS account? I can't access that article.
And for the rest of you who like myself are'nt interested in this longwinded synthesis, there may be a nugget at the end of the article on the O-demethylation of codiene.
(Chief Bee)
10-22-02 02:14
No 371039
../rhodium/pdf /morphine.synt
../rhodium/pdf /morphine.synt