(Hive Bee)
12-29-02 13:46
No 393799
(Rated as: excellent)
Rhodium, I think this might be something for your page.
Oxidation of Hydroquinones, Catechols, and Phenols using Ceric Ammonium Nitrate and Ammonium Dichromate Coated on Silica: An Efficient and Convenient Preparation of Quinones
Oxidation of Hydroquinone by the Cerium(IV)/SiO2 Reagent; Typical Procedure
Hydroquinone (0.11g, 1 mmol) in dichloromethane (15mL) is added with stirring to cerium(IV)/SiO2 reagent [6.94g, 2.1 mmol of cerium(IV)]. The mixture was stirred for 5 minutes and filtered. The residue is washed with dichloromethane (2 × 15mL). Evaporation of solvent from the combined filtrates gives product (0.106g) as a bright yellow crystalline solid which is found to be pure p-benzoquinone by 1H-N.M.R. (authentic sample from Aldrich). It is furthes purified by recrystallization from ether; mp 116° C.
Repetition of the oxidation on a 5 mmol scale yields 0.525g of p-benzoquinone.
Oxidation of Hydroquinone by the Chromium(VI)/SiO2 Reagent
Hydroquinone (0.11g, 1 mmol) in dichloromethane (10mL) is added with stirring to the chromium(VI)/SiO2 reagent [2.07g, 2.4 mmol of chromium(VI)]. The mixture is stirred for 5 minutes and filtered. The residue is washed with dichloromethane (2 × 15mL). Evaporation of solvent from the combined filtrates gave pure p-benzoquinone (0.104g). A 5 minute reaction with 5 mmol of hydroquinone yields 0.52g of pure p-benzoquinone.
Cerium(IV)/SiO2 and Chromium(VI)/SiO2 Reagents
To an efficiently stirred solution of ceric ammonium nitrate (20g) in MeOH (200mL), dichloromethane (200mL) followed by silica (200g, Baker Analyzed, 40-140 mesh) is added. The mixture is stirred for additional 15 minutes and the solvents are evaporated on a rotavapor. The resulting solid is dried for 3 h at 55° C on rotavapor, when free-flowing yellow powder is obtained. At this stage the yellow powder of cerium(IV)/SiO2 reagent weights 221.2g, suggesting that 1.2g of the solvent is retained. It is used as such for the oxidations. For this reagent 6.06g contain 1 mmol of cerium(IV).
A similar procedure using ceric ammonium nitrate (40g), silica (200g), MeOH (300mL) and dichloromethane (200mL) gives the 20% cerium(IV)/SiO2; yield: 242g [3.32g of the reagent contain 1 mmol of cerium(IV)].
Ammonium dichromate (40g) is dissolved in warm (50° C) 50:1 MeOH/H2O (600mL MeOH and 12mL H2O) and to this silica (200g) is added with stirring. After 10 minutes, the solvent is evaporated and the residue is dried for 10 h at 55° C on rotavapor. The 20% chromium(VI)/SiO2 reagent is obtained as a free-flowing orange powder; yield: 250.4g [0.79g of the reagent contain 1 mmol of chromium(VI)].
Reference: Synthesis, 1985, No. 6/7, pp 641
I'm dreaming of the white crystals.
(Hive Addict)
12-29-02 14:28
No 393802
This process certainly has the advantage of time, only 5 minutes
There are no reasons. Who needs reasons when you've got heroin?
(Hive Bee)
06-03-04 18:37
No 511243
(Rated as: excellent)
Aerobic oxidation of hydroquinones to quinones catalyzed by VO(acac)2
Der-Ren Hwang, Chang-Ying Chu, Sheng-Kai Wang, and Biin-Jiun Uang
Synlett, 1999, 1, 77-78

Abstract: In the presence of a catalytic amount of VO(acac)2, oxydation of hydroquinone and its derivatives with molecular oxygen at room temperature gave the corresponding quinones in moderate to high yields.
Key words: hydroquinones, quinones, oxidation, molecular oxygen, vanadyl acetylacetonate, vanadium
[...]
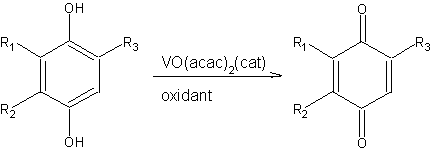 (2)
(2)Table 1. Oxidative dehydrogenation of hydroquinones to quinone derivatives catalyzed by VO(acac)2a
| Hydroquinone | R1 | R2 | R3 | time, h | product | yield, % |
| 2a | H | H | H | 10 | 2ab | 75 |
| 3a | Me | H | H | 14 | 3bc | 76 |
| 4a | Me | Me | H | 15 | 4bb | 76 |
| 5a | Me | Me | Me | 14 | 5bb | 97 |
| 6a | tBu | H | H | 10 | 6bc | 92 |
| 7a | Ph | H | H | 20 | 7bc | 88 |
| 8a | Br | H | H | 25 | 8bb | 72 |
| 9a | SPh | H | H | 12 | 9bc | 85 |
a These reactions were run with 10 mol% VO(acac)2 in CH2Cl2 at room temperature under 1 atm O2
b This compound was confirmed with the data reported in reference 9
c This compound was confirmed with the data reported in reference 11
[...]
A typical procedure follows: A stirred mixture of hydroquione 5a (380 mg, 25 mmol), and VO(acac)2 (66.3 mg, 0.1 mmol) in dichloromethane (25 ml) was exposed to an atmospheric pressure of molar oxygen at room temperature for 14 h. The mixture was then filtered through a short column of silica gel eluted with ethyl acetate (100 ml). The filtrate was concentrated and purified by column chromatography (SiO2, hexane : ethyl acetate = 10 : 1) to afford quinone 5b (362 mg, 97%, mp 29°C; lit9 31-32 °C)
[...]
References and notes
(9) Bosch, E.; Rathore, R.; Kochi, J. K. J. Org. Chem. 1994, 59, 2529
[...]
(11) Costantini, C., d'Ischia, M.; Prota, G. Synthesis 1994, 1399
The first line (oxidation of 2a to 2a and not 2b) is most likely a typo, as the authors write: "However when hydroquinone 2a was reacted with molecular oxygen in the presence of a catalytic amount of VO(acac)2 in dichloromethane at room temperature, quinone 2b was obtained in 75% yield after column chromatographic purification of the resulting mixture (eg.2)."
Vanadyl acetylacetonate, 100 g, ~55 $
The tendency is to push it as far as you can