01-07-03 14:19
No 396122
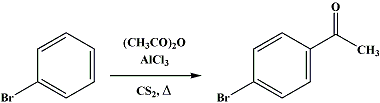
Procedure
In a 5-l. round-bottomed, three-necked flask, fitted with a mechanical stirrer, separatory funnel, and reflux condenser connected with a gas absorption trap (Fig. 7 on p. 97) for disposing of the evolved hydrogen chloride, is placed 392 g. (2.5 moles) of bromobenzene in 1-l. of dry carbon disulfide (Note 1). To this is added 750 g. (5.6 moles) (Note 2) of anhydrous aluminum chloride. The mixture is heated on a steam bath until gentle refluxing starts, and then 204 g. (2 moles) of acetic anhydride (Note 3) is added slowly through the dropping funnel. The time of addition is about one hour. Gentle refluxing should be continued throughout the time of addition of the anhydride and for one hour afterward. The reaction is accompanied by a copious evolution of hydrogen chloride which does not entirely cease even after this subsequent heating.
A condenser is attached to one of the side necks, and the carbon disulfide is distilled without removing the flask from the steam bath. After the removal of the solvent (Note 4), the reaction mixture is allowed to cool somewhat (Note 5); but while it is still warm, it is poured slowly with stirring over cracked ice to which hydrochloric acid has been added. In this way only a small amount of the aluminum chloride addition product remains in the flask. This is decomposed with ice and hydrochloric acid and added to the main product. The volume is now about 5 l. Each 2-l. portion is extracted twice with 300-cc. and 200-cc. portions of benzene or ether. The extracts are combined and washed twice with water, once with 10 per cent sodium hydroxide solution, and twice more with water. The final water washings should be practically colorless and the separation sharp, any precipitate being allowed to separate with the water.
The extract is dried for one hour with about 30 g. of calcium chloride and filtered, and the solvent is distilled from a steam bath. The residue is distilled under reduced pressure with a short column (p. 130). Some low-boiling material comes over first, and then the temperature rises rapidly. If care has been taken in the water decomposition to remove all the aluminum salts (Note 6), the product comes over water-white and crystallizes to a white solid melting at 4950.5°. The yield is 340395 g. (6979 per cent of the theoretical amount) of a product boiling over a 3° range (Note 7) and (Note 8). On redistillation the boiling point is 117°/7 mm.; 129130°/15 mm.; and 255.5°/736 mm.
Under the same general conditions, satisfactory yields of other acetophenones may be obtained. Thus, from 281 g. of chlorobenzene, 750 g. of aluminum chloride, and 205 g. of acetic anhydride, a consistent yield of 285300 g. (7478 per cent of the theoretical amount) of p-chloroacetophenone, boiling at 124126° /24 mm. and melting at 2021°, is obtained. Similarly, acetophenone may be obtained in 7683 per cent yields, p-methylacetophenone in 8589 per cent yields, and p-methoxyacetophenone in 9094 per cent yields.
Organic Syntheses, CV 1, 109
Arylfluorodehalogenation: Post 380478 (pHarmacist: "p-F-benzaldehyde from p-Cl-benzaldehyde", Methods Discourse)
Accept No Imitations, There Can Only Be One; www.the-hive.ws
(Hive Bee)
01-07-03 14:28
No 396124
You can call me a sissy when it comes to ethers. CS2 is in another ball park. It is a good but butt nasty solvent. Even steam baths are known to ignite this MF. They weren΄t conservative with the AlCl3 either.
Catalytic hydrogenation freak
(Hive Bee)
01-07-03 14:50
No 396127
I wouldn't fancy working with CS2, and our environment will appreciate it if we don't use it either - the products of combustion are not particualrly nice..
Would this procedure work using a halobenzene, acetyl chloride and (less!) aluminum chloride, in DCM? I have got a 90% yield using this procedure on plain benzene with butyryl chloride. Will the aromatic halide survive these conditions? I expect it will since it manages the excess of AlCl3 in the original post.