(One Remarkable HyperLab Bee)
01-13-04 14:23
No 482172
(Rated as: excellent)
The article requested by Manichi. The original text can be found here (http://hyperlab.0catch.com/ZhOrgKh_1996
I have no access to the English version of the journal requested, so the translation below is mine.
See Post 207554 (Antoncho: "An easy OTC 2,5-diMeO-phenylmercaptan", Novel Discourse),
Post 360747 (Rhodium: "2C-T-x Precursors: Ar-I + R-SH -> Ar-S-R", Novel Discourse), and
Post 434950 (Chimimanie: "On 2C-T-x precursors", Novel Discourse) for related material.
An alternative synthesis of 2-hydroxy-5-methoxythiophenol is described in PiHKAL #167: 4T-MMDA-2 (http://www.erowid.org/library/books_onl
Synthesis of Aminomethyl Derivatives of 2-Hydroxy-5-methoxythiophenol
M. M. Movsumzade, N. A. Novruzova, and Sh. R. Aliev
As a continuation of our previous studies concerning the synthesis of o-hydroxythiophenols and their derivatives [
Our preliminary experiments showed that the interaction of alkoxyphenols with sulfur monochloride is accompanied by fast side reactions which most often lead to the formation of undesired resinous products of unknown composition. That is why the synthesis of mercapto derivatives of alkoxyphenols presents certain difficulties. Hence, examination of the reaction of sulfur monochloride (S2Cl2) with 4-methoxyphenol I and elucidation of the optimum conditions for the synthesis of 2-hydroxy-5-methoxythiophenol II and its derivatives are needed. Using the reaction of the thiophenol II with various alkylating agents, many S-derivatives can be synthesized, the Mannich aminomethylation products being of particular interest for science and industry.
Reaction of 4-methoxyphenol I with sulfur monochloride with subsequent reduction of the disulfide III resulted in the thiophenol II.
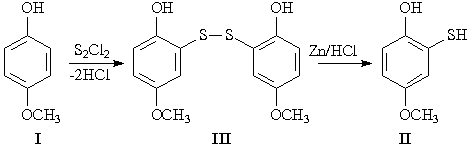
It was established that in the interaction of sulfur monochloride with 4-methoxyphenol I the best results are achieved when the reaction is conducted in benzene at 40-45 °C.
A series of aminomethyl derivatives IVa-d was synthesized by aminomethylation of the phenol II with formaldehyde and secondary amines.
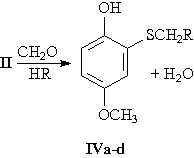
IV, R = N(C2H5)2 (a); N(C4H9)2 (b); morpholino (c); piperidino (d).
The reaction of the thiophenol II with formaldehyde and secondary amines was carried out under varying conditions. It was established that at 40-45 °C and with the ratio of formaldehyde, amine and the thiophenol being 1:1:1, aminomethylation occurs only at the -SH group to form the aminomethyl derivatives IVa-d. When the ratio of these substances is 2:2:1, both the -SH group and the position 3 are aminomethylated to form a mixture of mono- and bis(aminomethyl) derivatives with prevalence of the S-aminomethyl derivatives IVa-d. The mixture is difficult to separate.
At 75-80 °C in the presence of benzene with the ratio of reactants being 1:1:1, the S-aminomethylation products IVa-d are formed exclusively in 50-68% yield. The properties of the compounds obtained (II, IVa-d) are presented in the Table.
Yields and constants for 2-hydroxy-5-methoxythiophenol II and its aminomethyl derivatives IVa-d
| Compd | Yield, % | m. p., °C | d204 | n20D |
| II | 40 | 29-30a | - | - |
| IVa | 57 | - | 1.1007 | 1.5532 |
| IVb | 51 | - | 1.0165 | 1.5242 |
| IVc | 62 | 87b | - | - |
| IVd | 65 | 145b | - | - |
*The elemental analysis data are omitted. They are satisfactory except for IVb : C found 60.13, calcd 64.61 .
aRecrystallized from isooctane; b. p. 90-95 °C (0.5 mm Hg).
bRecrystallized from 2-propanol.
Since isolation of the aminomethyl derivatives by vacuum distillation is impossible because of thermal decomposition, in most cases they were isolated as hydrochloride salts. In case of formation of non-crystalline hydrochloride salts, the latter were treated with ammonia solution, converted to the corresponding bases, and extracted with diethyl ether. Crystalline aminomethyl derivatives were isolated by recrystallization from isooctane or 2-propanol.
The composition and the structure of the compounds synthesized were verified by elemental analysis and IR and 1H NMR spectroscopy [
In the 1H NMR spectrum of the thiophenol II all the signals characteristic of this structure are observed. Thus, the singlet at δ 2.4 ppm corresponds to the proton of the -SH group; the protons of the -OCH3 group are characterized by the signal at δ 4.0 ppm; the phenolic proton signal appears at δ 5.3 ppm, and the multiplet at δ 7.4-7.2 ppm corresponds to the protons of 1,2,4-trisubstituted benzene ring.
(---discussion of the IR spectra omitted---)
In the 1H NMR spectra of S-aminomethyl derivatives IVa-d the signal of the -SH group is missing, and the signals accounted for the newly introduced moieties appear.
Thus, in the 1H NMR spectra of all S-aminomethyl derivatives IVa-d the signals of the protons of the SCH2N moiety are observed in the region of δ 2-3 ppm. Besides, there are the signals of the hydrocarbon moieties of the NR2 group. Aliphatic moieties exhibit the signals of N(CH2)2 at δ 3-4 ppm, and the signals of CH2 and terminal CH3 groups (if those are present) at δ 1-2 ppm.
In the 1H NMR spectra of IVc, the signals of the protons of the OCH3 group are overlapped by the signals of morpholine O(CH2)2 to form a multiplet at δ 4.1 ppm; the N(CH2)2 signals appear as a multiplet at δ 3.0 ppm. In the 1H NMR spectra of IVd, there are the signals of the N(CH2)2 moiety at δ 2.5 ppm and the signals of (CH2)3 at δ 1.65 ppm.
(---discussion of the IR spectra omitted---)
Experimental part
IR spectra were recorded using a UR-10 spectrometer; 1H NMR spectra were obtained with a Varian 300 spectrometer relative to an internal reference of TMS.
2-Hydroxy-5-methoxythiophenol (II). To a solution of purified 4-methoxyphenol (I) (30 g) in benzene (100 ml) was added freshly distilled sulfur monochloride (16.2 g) over the course of 45 min at 40-45 °C. The reaction mixture was heated in a water bath until evolution of HCl ceased. Zinc dust (100 g) was added at 5-10 °C to the product obtained, followed by slow dropwise addition of conc. hydrochloric acid (500 ml). Then the reaction mixture was stirred for 1 h and heated in a water bath for 5 h. The mixture was extracted with benzene and washed with water. After evaporation of benzene, the residue was distilled under reduced pressure. Yield 40%, m. p. 29-30 °C.
Diethylaminomethyl 2-hydroxy-5-methoxyphenyl sulfide (IVa). To a 37% aqueous formaldehyde solution (5 g) was added diethylamine (4.6 g) dropwise at 20-25 °C, followed by the thiophenol II in diethyl ether (50 ml). The mixture was stirred for 4 h at 40-45 °C, treated with diethyl ether; the extract was washed with water and dried with sodium sulfate; dry hydrogen chloride gas was passed into the ethereal solution of the reaction product; the hydrochloride salt was washed several times with diethyl ether and finally decomposed by aqueous ammonia solution to isolate the free base of IVa.
The compounds IVb-d were obtained in an analogous manner.
References
1. A. M. Kuliev, Sh. R. Aliev, F. N. Mamedov, and Mirza Movsumzade, Zh. Org. Khim., 1976, 12(2), 426-431.
2. A. M. Zeynalov, F. N. Mamedov, Mirza Movsumzade, and A. K. Ibadzade, Zh. Org. Khim., 1979, 15(4), 816-820.
3. A. M. Kuliev, Sh. R. Aliev, F. N. Mamedov, and Mirza Movsumzade, Zh. Obshch. Khim., 1977, 47(11), 2492-2495.
4.A. M. Kuliev, Mirza Movsumzade, V. M. Kerimov, F. N. Mamedov, Zh. Org. Khim., 1982, 18(9), 1942-1945.
5. R. M. Silverstein, G. C. Bassler, T. C. Morrill, "Spectrometric Identification of Organic Compounds" (Russ. transl.).
6. L. J. Bellamy, "The Infrared Spectra of Complex Molecules" (Russ. transl.).