(Chief Bee)
03-13-04 07:14
No 494847
(Rated as: excellent)
This is a procedure which turns isosafrole to the diacetoxyglycol in 71% yield, and this can then of course easily be rearranged to MDP2P, maybe with Chromics novel high-yielding hydrochloric acid method, adapted to the preparation of MDP2P in Post 476334 (Chromic: "HCl is so good it's GOLDEN!", Chemistry Discourse) after a procedure published in the following article: Post 476342 (Rhodium: "Effect of various acids on pinacol rearrangement", Chemistry Discourse)
Electron-transfer Processes: Oxidation of
 - and
- and 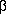 -Alkenylbenzenes by Peroxydisulphate in Acetic Acid
-Alkenylbenzenes by Peroxydisulphate in Acetic AcidAttilio Citterio, Claudio Arnoldi, Claudio Giordano, Graziano Castaldi
J. Chem. Soc. Perkin Trans. 1. 891-896 (1983) (../rhodium/pdf /diacetoxylat
Abstract
Oxidation of
 - and
- and 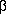 -unsaturated alkylbenzenes by peroxydisulphate in acetic acid gives side-chain acetoxylation with formation of the corresponding glycol diacetates and compounds (10), respectively. The reaction is catalysed by transition-metal salts, among which cupric acetate gives the best results. Generally, electron-releasing substituents on the benzene ring increase the yield and improve the selectivity. The same substrates are oxidized in water under Ag+ catalysis to the corresponding aldehydes. The different behaviour in the two solvents is ascribed to the difference in reactivity between the primary oxidation products and the starting olefin, whereas the initial oxidation step is suggested to occur in both cases via an electron-transfer process from the olefin to the sulphate radical anion.
-unsaturated alkylbenzenes by peroxydisulphate in acetic acid gives side-chain acetoxylation with formation of the corresponding glycol diacetates and compounds (10), respectively. The reaction is catalysed by transition-metal salts, among which cupric acetate gives the best results. Generally, electron-releasing substituents on the benzene ring increase the yield and improve the selectivity. The same substrates are oxidized in water under Ag+ catalysis to the corresponding aldehydes. The different behaviour in the two solvents is ascribed to the difference in reactivity between the primary oxidation products and the starting olefin, whereas the initial oxidation step is suggested to occur in both cases via an electron-transfer process from the olefin to the sulphate radical anion.Another reference related to this article has been posted in Post 487882 (Rhodium: "Oxidation of styrenes by peroxydisulfate-Cu(II)", Stimulants)
The Hive - Clandestine Chemists Without Borders