(Stranger)
04-26-04 07:23
No 503047
(Rated as: excellent)
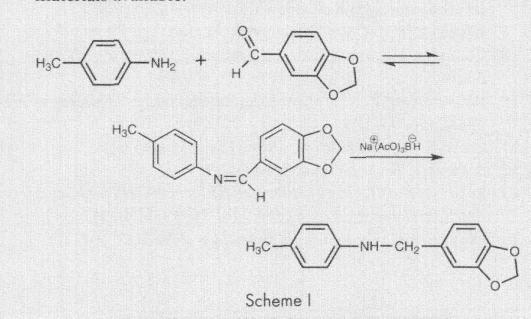
To a solution of 12.37 g (92.18 mmol, 1.0 equiv) of P2P in 100 mL of absolute THF in 500 mL Erlenmeyer flack was added a solution of 4.29 g (138.27 mmol, 1.5 equiv) of methylamine in 100 mL of absolute THF, then 11.1 g (184.36 mmol, 2.0 equiv) of glacial acetic acid and 29.31 g (138.27 g, 1.5 equiv) of STAB (sodium triacethoxyborohydride) in small (about 1-2 g) portions. The mixture warmed up to approx 50-60°C and foamed a bit. This milky-white light-viscous mixture was vigorously stirred for 2 h, the solvent was removed under reduced pressure. Water solution of alkaline a (50 mL) was added carefully into white residue and methamphetamine base was extracted with ethylacetate, organic layer was washed with brine dried over Na2SO4 and concentrated under reduced pressure. Yield – 13.5 g (98%) as light-yellow oil with good purity (according NMR and LC-MS). For additional purification, if it necessary, this oil can be purified via HCl-salt.
Notes:
I did this procedure himself - the quality of product was confirm by spectral data. But there is one negative element - STAB is quite expensive - about $100 for 100 g.
(Newbee)
04-26-04 11:47
No 503091
Sodium triacetoxyborohydride can be made from sodium borohydride and acetic acid in an aprotic solvent. The article linked to below presents many uses of acyloxyborohydrides.
Sodium borohydride in carboxylic acid media. A review of synthetic utility of acyloxyborohydrides.
G.W. Gribble & C.F. Nutaitis
Org. Prep. Proced. Int. 17(4-5), 317-384 (1985) (../rhodium/pdf /borohydride.
(Chief Bee)
04-26-04 14:29
No 503100
(Rated as: excellent)
ACS Symposium Series 641: Reductions in Organic Synthesis - Recent Advances and Practical Applications
Ed. Ahmed F. Abdel-Magid, 1996, American Chemical Society, Washington, DC
Sodium Borohydride and Carboxylic Acids: A Novel Reagent Combination (Chapter 11, pp. 167-200) (../rhodium/pdf /redamin.stab
Gordon W. Gribble
Abstract
The combination of sodium borohydride (NaBH4) and carboxylic acids – sodium acyloxyborohydrides – represents a remarkably versatile and powerfully efficient synthetic tool. This reagent manifold, the reactivity of which can be controlled depending on the nature and number of acyloxy groups, reduces and N-alkylates indoles, quinolines, isoquinolines, related heterocycles, imines, enamines, oximes, enamides, and similar functional groups. It reduces amides and nitriles to amines in the presence of esters, aryl alcohols and ketones to hydrocarbons, aldehydes to alcohols in the presence of ketones, and β-hydroxyketones to 1,3-diols stereoselectively. This reagent is also an extraordinarily useful methodology for the N-alkylation of primary and secondary amines, in a reaction sequence that is believed to involve sequential reduction of the carboxylic acid to the corresponding aldehyde followed by a standard reductive amination process. Frequently, the monoalkylation of primary amines can be achieved. The use of sodium cyanoborohydride (NaBH3CN) militates against N-alkylation, and, for example, the union of NaBH3CN/HOAc cleanly reduces indoles to indolines sans alkylation. Depending on the circumstances and conditions, alkenes can be hydroborated, esters and carboxylic acids can be reduced to alcohols, and arenes can be induced to undergo the Baeyer condensation. No other chemical system can boast of such amazing flexibility!
____ ___ __ _
ACS Symposium Series 641: Reductions in Organic Synthesis - Recent Advances and Practical Applications
Ed. Ahmed F. Abdel-Magid, 1996, American Chemical Society, Washington, DC
Use of Sodium Triacetoxyborohydride in Reductive Amination of Ketones and Aldehydes (Chapter 12, pp. 201-216) (../rhodium/pdf /redamin.stab
Ahmed F. Abdel-Magid and Cynthia A. Maryanoff
Abstract
Herein we present an overview of the use of sodium triacetoxyborohydride in the reductive amination of ketones and aldehydes, with an emphasis on scope. In general, this is an extremely useful reagent and experimental conditions are convenient and simple. Alicyclic and cyclic ketones furnish excellent yields of secondary and tertiary amines. Where diastereomer formation is possible, this reagent is more sterically demanding than sodium cyanoborohydride, and higher selectivity is often observed, especially from bicyclic ketones. Acid sensitive groups in the molecule are unaffected under normal reaction conditions. Hydrazines are reductively alkylated with ketones to furnish monalkyated products. Ketoesters are reductively aminated with primary and secondary amines. The initial products from γ- and δ-ketoesters or acids cyclize to the lactams in a tandem reductive cyclization procedure. The combination of NaBH(OAc)3/CF3CO2NH4 furnishes primary amines in high yields. Weakly basic amines demonstrate the clear advantage of this reagent, as they undergo reductive alkylation in high yields with ketones and aldehydes. Non-basic amines and sulfonamides furnish high yields of monoalkyl products with most aldehydes.
The Hive - Clandestine Chemists Without Borders
(Stranger)
04-26-04 16:29
No 503129
Thank you for links - I am going to investigate it thoroughly.
I did this reaction many times with big scale of different ketones and aldehydes with some compicated amines - it works great in ANY cases and exactly the same procesdure is using in combinatorial chemistry. I just (practically) expand it for our cases.
(Chief Bee)
08-08-04 22:50
No 524301
(Rated as: excellent)
Direct Reductive Alkylation of Amino Acids
Synthesis of Bifunctional Chelates for Nuclear Imaging
Murali K. Levadala, Sangeeta Ray Banerjee, Kevin P. Maresca, John W. Babich, Jon Zubieta
Synthesis 1759-1766 (2004) (../rhodium/pdf /stab.amino.a
Abstract
A family of effective bifunctional chelators for technetium- and rhenium-based radiopharmaceuticals was conveniently synthesized in high yields through direct reductive N-alkylations of amino acids and their analogues with aldehydes, using NaBH(OAc)3 as an efficient reagent. The mono-, di-, tetra- and even mixed alkylated amino acid derivatives were all prepared in one-pot synthesis.
In the case of amino acids, the reductive alkylation with aldehydes is generally performed after protection of the acid functionality as the ester, [17] rendering this a cumbersome three-step procedure. The reports that detail the reductive alkylation of amino acids with an open acid functionality (direct) are surprisingly few. The preferred reagent for this purpose is NaCNBH3 [18] , while NaBH4 [19] , H2/C-Pd(OH)2, [20] and NaHTe [21] have occasionally been used. Although NaCNBH3 is an excellent reagent, it is relatively toxic and the isolated products are often contaminated with cyanide residue. One limitation is that the reactions are restricted to polar medium, which is sometimes sluggish and often mitigated by poor yields. Furthermore, the scope of the substrate is limited in heterogeneous hydrogenations as many functional groups, such as nitro, alkene, alkyne, OBn etc., are susceptible under the reaction condition. The use of NaBH4 necessitates the preformed imine to avoid the reduction of aldehyde, and also alkaline medium, thereby limiting its practicality. Abdel-Magid et al. [22] have recently demonstrated that NaBH(OAc)3 can effectively be used as a mild reagent in the reductive amination of aldehydes and ketones with shorter reaction time and excellent yields. It is surprising, that with the exception of a single example, there are no major reports of use of NaBH(OAc)3 with free amino acids, [23e] even though there are several reports in the literature for the corresponding amino acid esters. [23] We sought to exploit the above method to provide direct access to ligands useful for applications to nuclear medicine.
We noted literature reports of reductive alkylation of phenylalanine with pyridine-2-carboxaldehyde for which a 21% yield with NaCNBH3 had been obtained for the monoalkyl derivative. [18b] We have now investigated the dialkylation of the same system with NaBH(OAc)3 in 1,2-dicholorethane (DCE) (Scheme [1] ). The reaction was complete within two hours, and the product 6 was isolated in 78% yield. Since DCE has been used in similar reactions, we employed the same medium, despite the insolubility of amino acids in DCE. It is noteworthy that the reaction was homogeneous in DCE despite the insolubility of amino acids while a suspension-like mixture was evident in the polar solvent MeCN. The rate of the reaction was significantly faster in chlorinated solvents, DCE and CH2Cl2 compared to THF and MeCN.
By appropriate stoichiometric manipulation of the amino acids and aldehydes, the mono-, di-, and even tetraalkylated amino acid derivatives could be obtained in good yields. In the case of tetraalkylation, the lysine dihydrochloride salt was treated with 4.5 equivalents of pyridine-2-carboxaldehyde and NaBH(OAc)3 (5 equivalents) to afford the tetrapyridyl derivative 10 in 79% yield, along with a small amount of the reduced alcohol of the aldehyde. It is noteworthy that four alkylation steps are performed in one-pot in the case of such tetraalkyl amino acids, an observation consistent with the direct use of the amino acid hydrochloride salt as starting materials without any need for prior neutralization or base addition to the reaction, at the expense of a slight excess of aldehyde (0.5 equiv).
The simplified workup procedure involves the addition of water to quench the reaction followed by extraction into the organic phase. The methodology was also amenable to large scale synthesis, as we have successfully scaled up the reductive alkylation of amino acids (compound 1) to a 10 g scale.
Encouraged by the above results, we then investigated the monoalkylation of similar systems with stoichiometric amounts of aldehyde and amine (compounds 12, 13). Unfortunately, the reaction resulted in the predominant formation of dialkylated product. However, it was subsequently found that the dialkylation could be significantly suppressed by forming the imine [25] in situ, i.e., refluxing the mixture of amino acid and aldehyde in DCE for 10 minutes under inert atmosphere (Scheme [2] ), followed by treatment with NaBH(OAc)3 at ambient temperature. This enabled us to isolate the monoalkylated products 12 and 13 in good yields with the formation of dialkylated products reduced to 10-15%. This small amount of dialkylation may be explained in light of the observation for amino esters by Abdel-Magid et al. [23c] that the monoalkylated product itself would add to the imine to form a dialkyliminium ion, which could then be reduced by NaBH(OAc)3 to afford the dialkylated product. We found that other aprotic solvents CH2Cl2, THF and MeCN along with protic solvents such as methanol were equally effective for the formation of imine.
Abstract
Dialkylation Reactions; Typical Procedure
To a mixture of Fmoc-l-lysine (10.0 g, 27.1 mmol) and NaBH(OAc)3 (14.4 g, 67.95 mmol) in DCE (150 mL), was added 2-pyridinecarboxaldehyde (6.4 g, 57.0 mmol) in DCE (15 mL) at 0 °C under argon. The suspension was stirred at r.t. for 1 h. The reaction mixture was decomposed with H2O (100 mL) and diluted with CHCl3 (100.0 mL). The separated organic layer was washed with H2O and brine, dried (Na2SO4) and concentrated under reduced pressure. The residue was purified through a pad of silica gel using MeOH-CHCl3 (1:6) as eluent to provide the dipyridylmethyl derivative of Fmoc-l-lysine (12.85 g, 86%).
Dialkylation Reactions; Typical Procedure
To a mixture of Fmoc-l-lysine (10.0 g, 27.1 mmol) and NaBH(OAc)3 (14.4 g, 67.95 mmol) in DCE (150 mL), was added 2-pyridinecarboxaldehyde (6.4 g, 57.0 mmol) in DCE (15 mL) at 0 °C under argon. The suspension was stirred at r.t. for 1 h. The reaction mixture was decomposed with H2O (100 mL) and diluted with CHCl3 (100.0 mL). The separated organic layer was washed with H2O and brine, dried (Na2SO4) and concentrated under reduced pressure. The residue was purified through a pad of silica gel using MeOH-CHCl3 (1:6) as eluent to provide the dipyridylmethyl derivative of Fmoc-l-lysine (12.85 g, 86%).
Mixed-Alkylation Reactions; Typical Reaction
A solution of Boc-d-lysine (2.0 g, 8.12 mmol) and 2-pyridinecarboxaldehyde (0.87 g, 8.12 mmol) in DCE (10 mL) was refluxed for 10 min under argon. The reaction mixture was cooled to 0 °C, and treated sequentially with NaBH(OAc)3 (4.30 g, 20.3 mmol) and 2-thiazolecarboxyaldehyde(0.91 g, 8.12 mmol). The reaction mixture was stirred at r.t. until the completion of reaction and purified as mentioned above to obtain the mixed derivative of Boc-d-lysine (3.39 g, 75%) along with 12% of the bis-alkyl derivative as a side product.
This procedure might be useful for the N-methylation of phenylalanine (for later reduction to methamphetamine) as discussed in Post 512010 (java: "Question: methylating the amine in Phenylalanine", Stimulants) or possibly even for the N-methylation of plain amphetamine to form methamphetamine, as mentioned in Post 450039 (Kinetic: "Methylation", Stimulants).
This procedure is also very likely to be just as good for the N,N-dimethylation of tryptamine to DMT as the currently leading method: Post 435056 (Rhodium: "DMT from Tryptamine/NaBH3CN/37% HCHO", Tryptamine Chemistry)
Another use for this procedure could be the N,N-dialkylation of tryptophan to the correspoding N,N-Dialkyl-tryptophan, which is a simple decarboxylation away from a range of N,N-Dialkyltryptamines - a route discussed in Post 445300 (Vitus_Verdegast: "Can J Chem 80: 779-788 (2002)", Tryptamine Chemistry)
Also, from reference [19] in the article: NaBH4 reductive alkylation of aminoacids:
Synthesis of novel 1,10-phenanthroline-2,9-bis-α-amino
Wang Z-M.; Lin H-K.; Zhou Z-F.; Zhu S-R.; Liu T-F.; Chen Y-T., J. Chem. Res. (S), No. 4, pp. 170-171 (2000)
Abstract
Starting from 2,9-dimethyl-1,10-phenanthroline, the syntheses of eight novel 1,10-phenanthroline-2,9-bis-α-amino
The other reference, Andruszkiewicz, R.; Pol. J. Chem. 62, 257 (1988), may be written in polish, but I'm not sure.
The Hive - Clandestine Chemists Without Borders
08-08-04 23:21
(Rated as: redundant)
(Chief Bee)
09-14-04 20:40
No 531309
Sodium Triacetoxyborohydride
S. D. Burke & R. L. Danheiser (Ed.)
Handbook of Reagents for Organic Synthesis: Oxidizing and Reducing Agents, pp. 429-432 John Wiley & Sons, Chichester, UK (1999) (../rhodium/pdf /stab-handboo
The Hive - Clandestine Chemists Without Borders
(Stranger)
09-16-04 16:13
No 531610
(Rated as: excellent)
Reductive Amination of Aldehydes and Ketones with Sodium Triacetoxyborohydride. Studies on Direct and Indirect Reductive Amination Procedures
(Ahmed F. Abdel-Magid, Kenneth G. Carson, Bruce D. Harris, Cynthia A. Maryanoff, and Rekha D. Shah;
J.Org.Chem. 1996, 61 (3849-3862))
Abstract
Sodium triacetoxyborohydride is presented as a general reducing agent for the selective amination of aldehydes and ketones. Procedures for using this mild and selective reagent have been developed for a wide variety of substrates. The scope of the reaction includes alipathic acyclic and cyclic ketones, aliphatic and aromatic aldehydes, and primary and secondary amines including a variety of weakly basic and nonbasic amines. Limitations include reactions with aromatic and unsaturated ketones and of some sterically hindered ketones and amines. 1,2-Dichloroethane (DCE) is the preferred reaction solvent, but reactions can also be carried out in tetrahydrofuran (THF) and occasionally in acetonitrile. Acetic acid may be used as catalyst with ketone reactions, but it is generally not needed with aldehydes. The procedure is carried out in the presence of reducible functional groups such as C-C multiple bonds and cyano and nitro groups. Reactions are generally faster in DCE than in THF, and in both solvents, reactions are faster in the presence of AcOH. In comparison with other reductive amination procedures such ans NaBH3CN/MeOH, borane-pyridine, and catalyic hydrogenation, NaBH(OAc)3 gave consistently higher yields and fewer side products. In the reductive amination of some aldehydes with primary amines where dialkylation is a problem we adopted a stepwise procedure involving imine formation in MeOH followed by reduction with NaBH4.

I hope this wasn't posted before - but it looked interesting to me, especially as it is a general overview on carboxyborohydrides and a follow-up from the same author of two of the articles mentioned above: A.F. Abdel-Magid. (They say that for reductive aminations of ketones, DCE is superior to THF solvent BTW).
indole_amine
(Stranger)
09-16-04 16:27
No 531611
(Rated as: excellent)
Sodium borohydride in carboxylic acid media: a phenomenal reduction system
(Gordon W. Gribble, Chem.Soc.Rev. 1998, 27 (395-404))
The union of sodium borohydride and carboxylic acids has yielded an amazingly versatile and efficient set of reducing reagents. These acyloxyborohydride species reduce and N-alkylate indoles, quinolines, isoquinolines, related heterocycles, imines, enamines, oximes, enamides, and similar functional groups. They reduce amides and nitriles, aryl alcohols and ketones, aldehydes in the presence of ketones, and beta-hydroxyketones to 1,3-diols stereoselectively. This reagent is also extraordinarily useful for the N-alkylation of primary and secondary amines with aldehydes and ketones in a novel reductive amination process.

Again from an already cited author; Gordon W. Gribble gives examples for N-alkylation of tryptamine (and other amines), using triacetoxyborohydride/HOAc in DCE with 87% yield, further several reductions of imines and oximes are discussed...
(I wonder if G.W. Gribble copies A.F. Abdel-Magid or other way round; because this paper is essentially the same as the first Abdel-Magid article that was mentioned in this thread! Even the same drawings...)
indole_amine
(Stranger)
09-17-04 05:18
No 531724
(Rated as: already posted)
Synthetically Useful Reactions with Metal Boride and Aluminide Catalysts
(Bruce Ganem, John O. Osby; Chem.Rev. 1986, 86 (763-780))
"(...)In the past 40 years, metal hydrides, particularly sodium borohydride and lithium aluminum hydride, have emerged as preeminent reducing agents in modern organic chemistry. These are extraordinarily versatile reagents capable of reducing most functional groups. Moreover by attaching organic ligands at boron or aluminum or changing the metal counterion, one can modulate the scope, regio-, and stereoselectivity of such reductions. Literally hundreds of substituted boron and aluminum hydrides have been described in the chemical literature and dozens are now commercially available. More recently, transition metal salts have been used as catalysts or additives in conjunction with NaBH4 and LiAlH4 to modify or enhance the properties of these reagents. Nearly every conceivable combination of salt and hydride has been investigated with the concomitant development of many useful new synthetic methods(...)"
Contents
I Introduction
A Background
B Catalyst preparation
1 Borides
2 Aluminides
C Catalyst Composition and Properties
1 Borides
2 Aluminides
D Useful Reducing Systems
II Reactions Involving Borides and Aluminides
A Hydrogenation of Alkenes and Alkynes
1 Borides
2 Aluminides
B Reduction of Arenes
C Reduction of Halides
1 Borides
2 Aluminides
D Reduction of Nitriles
E Reduction of Nitro Compounds
1 Nitroarenes
2 Nitroalkanes
F Reduction of Other Nitrogenous Functional Groups
1 Amides
2 Oximes
3 Azoxy, Azo, Nitroso Compounds, and Hydroxylamines
4 Isoxazolidines
G Deoxygenation Reactions
1 Sulfoxides
2 Phosphine Oxides
3 Ethers and Esters
4 Aryl Ketones
H Desulfurization Reactions
I Miscellaneous Reactions
1 Formation of Amine Boranes
2 Hydroboration
3 Epoxide Opening
4 Deselenation
J Conclusion
../rhodium/pdf /metal.boride.
indole_amine
(Stranger)
09-17-04 18:05
No 531798
I didn't know this paper was already "republished" here before
But using the search engine, I wasn't able to relocate this article (search string was "Ganem Osby"), therefore it would be nice if you could provide me with a link; so that I can edit my above post and replace the uploaded pdf with the one already present (there is no need to have such a big file archieved twice IMO
Thanks in advance!
indole_amine
(Chief Bee)
09-17-04 18:43
No 531807
Finding Articles: Post 445019 (Rhodium: "How to find already posted articles in TFSE", General Discourse) and Post 445935 (Rhodium: "UTFSE tips", General Discourse)
The Article: Post 380674 (Barium: "Chem. Rev. 1986, 86, 763-780 Synthetically useful", Novel Discourse)
The Hive - Clandestine Chemists Without Borders
(Stranger)
09-18-04 04:19
No 531894
(Rated as: good read)
A simple secondary amine synthesis: Reductive amination using sodium triacetoxyborohydride
Merle W. Carlson, James T. Ciszewski, Micah M. Bhatti, Wesley F. Swanson, Anne M. Wilson
Journal of Chemical Education. Easton: Feb 2000. Vol. 77, Iss. 2; pg. 270-271

Abstract
In modern organic synthesis, reductive amination is considered one of the principal ways to make secondary and tertiary amines. A detailed experimental procedure for the synthesis of secondary amines for a second-semester organic chemistry class is described.
...and the last lines:
"Acknowledgement
We would like to thank Butler University's 1998 spring organic chemistry classes for assisting with the preparation and testing of this lab."
Hmmm - lucky Butler 2nd-semester '98 students - lots of MDMA for their folks if they were clever during this "testing" period...
indole_amine
(Hive Bee)
09-18-04 05:18
No 531919
A Few questions, apparently you can DCM as your solvent of choice? And it appears that you can use amine salts? Now can some one devise a procedure for our most usefull ketones using Triacetoxyborohydride, dcm, and meam hcl?
(Stranger)
09-18-04 06:37
No 531934
DCM isn't the preferred solvent, but it should work, as water is rather soluble in it.
But amine salts cannot be used directly, they must be liberated by a stronger base like triethylamine or NaOH - and this reaction produces H2O, which is detrimental to imine formation.
Maybe it would be best to first form the imine in DCM using the amine hydrochloride and NaOH, together with molecular sieves or other inert drying agents, then separate from solids by suction filtration, add some acetic acid (to supress ketone reduction) and then add the NaBH(OAc)3 in small increments, let react for several hours at moderate temperature (reflux setup), destroy the excess acetoxyborohydride with sodium bicarbonate, add water and separate the organic layer, followed by extracting further with DCM.
Wash the combined organic extracts with H2O, then twice with satd. NaCl-soln., dry thoroughly (first with MgSO4, then over solid KOH pellets, overnight), remove solvent and distill the residue over a 40cm vigreux column under vacuum, dissolve the so obtained, pure amine base in cold, anhydrous Et2O (or DCM) and precipitate the hydrochloride by adding a slight molar excess of 5N HCl/IPA followed by cooling for several hours, then filtering precipitated hydrochloride, then rinse with dried, cold acetone, combine mother liquor and acetone washes and cool again to obtain a second crystallization, collect via suction filtration and recrystallize your both secondary amine hydrochloride crops from boiling EtOAc/Acetone 9:1 or similar.
Dry in vacuo over KOH pellets to remove solvent and water traces.
Yields should be in the 80% range (calculated from the mol. amount of your ketone), with a purity of around 99.9 percent, but this is of course just theoretical..
indole_amine
(Hive Bee)
09-18-04 07:06
No 531938
page 3860 procedure 1) direct reductive amination A) THF, Ch2Cl2 nad Ch3Cn may be used. C)Salts of amines may be use
(Stranger)
09-18-04 17:05
No 531993
J.Org.Chem. 61, 11 (p.3860)
"1) Direct Reductive Amination Methods
(...)
b) Acetic acid (1-2 molar equiv.) may be used in reactions of ketones but is not necessary with most aldehydes.
c) Reactions are normally carried out using the free amines; however, the amine salt may be used.
In this case, 1-2 equiv. of Et3N is added to the reaction mixture. The Et3N must be removed from basified product prior to salt formation."
As I already said: "But amine salts cannot be used directly, they must be liberated by a stronger base like triethylamine(...)"
If you use triethylamine (or any other tertiary amine), your methylamine will be liberated without water formation; with the cheaper variant NaOH, the neutralization of the acid part of your amine salts produces H2O - but in both cases, the amine is liberated from its salt prior to forming the imine.
And as the imine formation proceeds preferably under anhydrous conditions, triethylamine might be the better choice. NaOH is more available though, but makes the use of drying agents mandatory.
indole_amine
(Hive Bee)
11-01-04 20:41
No 539104
(Rated as: excellent)
Polymer-supported triacetoxyborohydride: a novel reagent of choice for reductive amination
(Sukanta Bhattacharyya, Sunil Rana, Owen W. Gooding, Jeff Labadie)
Tetrahedron Letters 44 (27), 2003, pp.4957-4960

Abstract
A novel polymer-supported triacetoxyborohydride reagent for reductive amination of aldehydes and ketones is reported. The bound reagent was found to be shelf-stable and provided broad scope and reactivity in reductive amination reactions under mild reaction conditions. Streamlined protocols for its application in reductive amination reactions using both primary and secondary amines are described.
indole_amine