2,5-Dimethoxyphenylacetone via Darzen Condensationby Barium[ Back to the Chemistry Archive ] 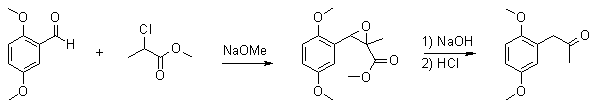
To a 500ml rb three-neck flask with a thermometer and a stir bar was added 16.6g (100 mmol) 2,5-dimethoxybenzaldehyde and 18.3g (150mmol) methyl 2-chloropropionate. 100ml MeOH was added to get the benzaldehyde into solution. While keeping the reaction mixture at 15°C, 8.1g (150 mmol) sodium methoxide in 50ml MeOH was added dropwise during 40min. When all alkoxide was added the reaction mixture was allowed to come to room temp and stir for another hour. The reaction mixture was then added to 10g (250 mmol) NaOH in 40ml water while keeping the temp at 20°C, then all was stirred at room temp over night. The next morning there was a thick white precipitate in the solution. To this 15% aq HCl was added until pH 3.5 was reached. This caused a clear yellow oil to fall out. The solution was heated on a waterbath at 65°C for two hours to complete the decarboxylation. The methanol was removed by distillation in a rotovap and the ketone was isolated by steam distillation. The distillate was extracted with 3x75ml toluene and the collected toluene phases dried over MgSO4. The toluene was removed by distillation in a rotovap leaving a clear yellow oil. Yield: 14.94g (76.9% yield) 2,5-dimethoxyphenylacetone
|